The human natural anti-αGal antibody targets common pathogens by broad-spectrum polyreactivity
- PMID: 33340093
- PMCID: PMC7968403
- DOI: 10.1111/imm.13297
The human natural anti-αGal antibody targets common pathogens by broad-spectrum polyreactivity
Abstract
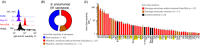
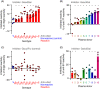
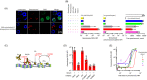
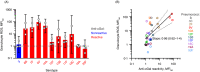
Naturally occurring antibodies are abundant in human plasma, but their importance in the defence against bacterial pathogens is unclear. We studied the role of the most abundant of such antibodies, the antibody against terminal galactose-α-1,3-galactose (anti-αGal), in the protection against pneumococcal infections (Streptococcus pneumonia). All known pneumococcal capsular polysaccharides lack terminal galactose-α-1,3-galactose, yet highly purified human anti-αGal antibody of the IgG class reacted with 48 of 91 pneumococcal serotypes. Anti-αGal was found to contain multiple antibody subsets that possess distinct specificities beyond their general reactivity with terminal galactose-α-1,3-galactose. These subsets in concert targeted a wide range of microbial polysaccharides. We found that anti-αGal constituted up to 40% of the total antibody reactivity to pneumococci in normal human plasma, that anti-αGal drives phagocytosis of pneumococci by human neutrophils and that the anti-αGal level was twofold lower in patients prone to pneumococcal infections compared with controls. Moreover, during a 48-year period in Denmark, the 48 anti-αGal-reactive serotypes caused fewer invasive pneumococcal infections (n = 10 927) than the 43 non-reactive serotypes (n = 18 107), supporting protection on the population level. Our findings explain the broad-spectrum pathogen reactivity of anti-αGal and support that these naturally occurring polyreactive antibodies contribute significantly to human protective immunity.
Keywords: antibodies; epitopes; flow cytometry; microbiota.
© 2020 John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no competing financial and commercial interests in relation to the manuscript.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

