Risk-efficacy balance of ulipristal acetate compared to surgical alternatives
- PMID: 33341097
- PMCID: PMC8359338
- DOI: 10.1111/bcp.14708
Risk-efficacy balance of ulipristal acetate compared to surgical alternatives
Abstract
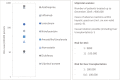
Aims: Uterine fibroids are benign tumours that cause various complaints. These complaints may significantly compromise quality of life, necessitating a clinical intervention in 25-50% of the affected women. Hysterectomy, myomectomy or embolization may offer symptomatic relief, but are costly, include a recovery period, can cause serious side-effects, sometimes fail to treat symptoms completely and are not always desired by patients. Ulipristal is a conservative long-term treatment that has a fibroid-volume decreasing effect, acceptable side-effects while preserving fertility and may be an alternative to surgical alternatives. Currently, ulipristal is investigated by the European Medicine Agency and suspended from marketing authorization because it may cause drug-induced liver injury (DILI). However, many drugs can cause severe DILI and prospective studies estimate 14-19 DILI cases/100 000 people.
Methods: This overview will discuss the risk-benefit balance between ulipristal and DILI, describe the safety-efficacy balance of ulipristal and its alternative treatments and the arguments that led to the suspension of its marketing authorization.
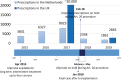
Results: Ulipristal may be associated with DILI resulting in a risk of severe liver injury in 1.5:100 000 patients and fatal liver injury in 0.1:100 000 patients. This risk needs to be weighed against the higher mortality risk of >1:1000 and higher incidence of severe complications after surgery.
Conclusion: The DILI risk of ulipristal is considerably lower than that of other medicines that are not suspended, nor need additional safety measures. When evaluating drugs and drug safety, risks that apply to the alternative nonpharmacological treatment options should be taken into consideration.
Keywords: chemical and drug induced liver injury; clinical pharmacology; hepatopharmacology; hysterectomy; leiomyoma; safety pharmacology; ulipristal acetate.
© 2020 The Authors. British Journal of Clinical Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Conflict of interest statement
The authors declare that they have no conflict of interest. The authors do not have any relation with Preglem SA, they do not participate in any advisory board related to Preglem SA and do not receive any funding from Gedeon Richter. M.A.M., W.J.F.H. and J.A.F.H. are currently performing a randomized–controlled trial that compares ulipristal in long‐term intermittent treatment with standard surgery (hysterectomy, myomectomy and uterine artery embolization): MYOMEX‐2 trial; Dutch Trial Register number: NTR6860; NL62638.029.18. This is a principle investigator initiated study, funded by NWO‐ZonMW.
Figures

References
-
- Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: Ultrasound evidence. Am J Obstet Gynecol. 2003;188(1):100‐107. - PubMed
-
- Stewart EA. Uterine fibroids. Lancet. 2001;357(9252):293‐298. - PubMed
-
- Parazzini F, Tozzi L, Bianchi S. Pregnancy outcome and uterine fibroids. Best Pract Res Clin Obstet Gynaecol. 2016;34:74‐84. - PubMed
-
- Singh SS, Belland L, Leyland N, von Riedemann S, Murji A. The past, present, and future of selective progesterone receptor modulators in the management of uterine fibroids. Am J Obstet Gynecol. 2017:563–572. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

