Global production capacity of seasonal and pandemic influenza vaccines in 2019
- PMID: 33341308
- PMCID: PMC7814984
- DOI: 10.1016/j.vaccine.2020.12.018
Global production capacity of seasonal and pandemic influenza vaccines in 2019
Abstract
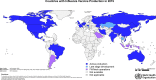
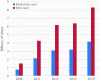
Vaccines will be an important element in mitigating the impact of an influenza pandemic. While research towards developing universal influenza vaccines is ongoing, the current strategy for vaccine supply in a pandemic relies on seasonal influenza vaccine production to be switched over to pandemic vaccines. Understanding how much vaccine could be produced, in which regions of the world and in what timeframe is critical to informing influenza pandemic preparedness. Through the Global Action Plan for Influenza Vaccines, 2006-2016, WHO promoted an increase in vaccine production capacity and monitors the landscape through periodically surveying influenza vaccine manufacturers. This study compares global capacity for production of influenza vaccines in 2019 with estimates from previous surveys; provides an overview of countries with established production facilities; presents vaccine production by type and manufacturing process; and discusses limitations to these estimates. Results of the current survey show that estimated annual seasonal influenza vaccine production capacity changed little since 2015 increasing from 1.47 billion to 1.48 billion doses with potential maximum annual influenza pandemic vaccine production capacity increasing from 6.37 billion to 8.31 billion doses. However, this figure should be interpreted with caution as it presents a best-case scenario with several assumptions which may impact supply. Further, pandemic vaccines would not be immediately available and could take four to six months for first supplies with several more months needed to reach maximum capacity. A moderate-case scenario is also presented of 4.15 billion doses of pandemic vaccine in 12 months. It is important to note that two doses of pandemic vaccine are likely to be required to elicit an adequate immune response. Continued efforts are needed to ensure the sustainability of this production and to conduct research for vaccines that are faster to produce and more broadly protective taking into account lessons learned from COVID-19 vaccine development.
Keywords: Capacity; Influenza; Pandemic; Production; Seasonal; Vaccine.
Copyright © 2020. Published by Elsevier Ltd.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

