Impaired autophagy: The collateral damage of lysosomal storage disorders
- PMID: 33341443
- PMCID: PMC7753127
- DOI: 10.1016/j.ebiom.2020.103166
Impaired autophagy: The collateral damage of lysosomal storage disorders
Abstract
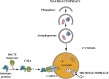
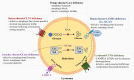
Lysosomal storage disorders (LSDs), which number over fifty, are monogenically inherited and caused by mutations in genes encoding proteins that are involved in lysosomal function. Lack of the functional protein results in storage of a distinctive material within the lysosomes, which for years was thought to determine the pathophysiology of the disorder. However, our current view posits that the primary storage material disrupts the normal role of the lysosome in the autophagic pathway resulting in the secondary storage of autophagic debris. It is this "collateral damage" which is common to the LSDs but nonetheless intricately nuanced in each. We have selected five LSDs resulting from defective proteins that govern widely different lysosomal functions including glycogen degradation (Pompe), lysosomal transport (Cystinosis), lysosomal trafficking (Danon), glycolipid degradation (Gaucher) and an unidentified function (Batten) and argue that despite the disparate functions, these proteins, when mutant, all impair the autophagic process uniquely.
Keywords: Autophagy; Batten disease; Cystinosis; Danon disease; Gaucher disease; Lysosome; Pompe disease.
Copyright © 2020 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors have nothing to disclose.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

