Nanotechnology in pulmonary medicine
- PMID: 33341460
- PMCID: PMC7746087
- DOI: 10.1016/j.coph.2020.11.002
Nanotechnology in pulmonary medicine
Abstract
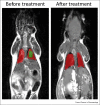
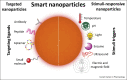
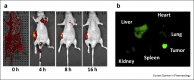
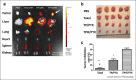
Nanotechnology in medicine-nanomedicine-is extensively employed to diagnose, treat, and prevent pulmonary diseases. Over the last few years, this brave new world has made remarkable progress, offering opportunities to address historical clinical challenges in pulmonary diseases including multidrug resistance, adverse side effects of conventional therapeutic agents, novel imaging, and earlier disease detection. Nanomedicine is also being applied to tackle the new emerging infectious diseases, including severe acute respiratory syndrome coronavirus (SARS-CoV), Middle East Respiratory Syndrome Coronavirus (MERS-CoV), influenza A virus subtype H1N1 (A/H1N1), and more recently, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). In this review we provide both a historical overview of the application of nanomedicine to respiratory diseases and more recent cutting-edge approaches such as nanoparticle-mediated combination therapies, novel double-targeted nondrug delivery system for targeting, stimuli-responsive nanoparticles, and theranostic imaging in the diagnosis and treatment of pulmonary diseases.
Keywords: Asthma; COVID- 19; Cystic fibrosis; Lung cancer; Nano carrier; Nanomedicine; Pneumonia; Respiratory disease; Tuberculosis.
Copyright © 2020 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures
References
-
- The burden of lung disease: the European Lung white book. 2011. https://www.erswhitebook.org/chapters/the-burden-of-lung-disease/ [cited 2020; Available from.
-
- Barnes P.J., et al. Barriers to new drug development in respiratory disease. Eur Respir J. 2015;45:1197–1207. - PubMed
-
- Doroudian M., et al. Nanotechnology based therapeutics for lung disease. Thorax. 2019;74:965. - PubMed
-
Comprehensive review discussing recent progress and development in clinical applications of nanotechnology in respiratory diseases. This paper covers a wide range of attempts in clinical trials employing nanotechnology for theraputic agent delivery, vaccination, and detection for the treatment of lung diseases.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

