Italian survey on the clinical management of non-small cell lung cancer patients during the COVID-19 pandemic: A lesson for the second wave
- PMID: 33341505
- PMCID: PMC7691849
- DOI: 10.1016/j.critrevonc.2020.103189
Italian survey on the clinical management of non-small cell lung cancer patients during the COVID-19 pandemic: A lesson for the second wave
Abstract
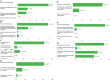
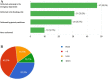
This study investigated the clinical management of non small cell lung cancer (NSCLC) patients during the first wave of coronavirus disease 2019 (COVID-19) outbreak in Italy. A 29-questions survey was sent to 95 Italian thoracic oncologists, with 77 % of them declaring significant changes in the outpatients management and treatment. The results of this survey pointed out a significant delay of lung cancer diagnosis along with a relevant reduction of patients' accrual within clinical trials. Telemedicine emerged as a valid support for patient-healthcare interactions. Therapeutic indications followed the guidelines for adjuvant chemotherapy and concurrent chemo-radiation. Clinical indications to first-line therapies were largely confirmed, while major changes regarded the selection of second line treatment options as well as the management of elderly population. This work may represent a valid source of information to improve the clinical management of NSCLC patients during second wave of COVID-19 pandemic.
Keywords: COVID-19; Lung cancer; Outpatient management; SARS‐CoV‐2; Survey.
Copyright © 2020 Elsevier B.V. All rights reserved.
Figures



Similar articles
-
Management of patients with cancer during the COVID-19 pandemic: The Italian perspective on the second wave.Eur J Cancer. 2021 May;148:112-116. doi: 10.1016/j.ejca.2021.01.040. Epub 2021 Feb 25. Eur J Cancer. 2021. PMID: 33743478 Free PMC article.
-
Impact of COVID-19 outbreak on cancer immunotherapy in Italy: a survey of young oncologists.J Immunother Cancer. 2020 Oct;8(2):e001154. doi: 10.1136/jitc-2020-001154. J Immunother Cancer. 2020. PMID: 33060148 Free PMC article.
-
[Expert recommendations on the management of patients with advanced non-small cell lung cancer during epidemic of coronavirus disease 2019 (Trial version)].Zhonghua Jie He He Hu Xi Za Zhi. 2020 Apr 12;43(4):297-301. doi: 10.3760/cma.j.cn112147-20200221-00138. Zhonghua Jie He He Hu Xi Za Zhi. 2020. PMID: 32125132 Chinese.
-
Optimizing lung cancer radiation treatment worldwide in COVID-19 outbreak.Lung Cancer. 2020 Aug;146:230-235. doi: 10.1016/j.lungcan.2020.05.029. Epub 2020 May 26. Lung Cancer. 2020. PMID: 32585497 Free PMC article. Review.
-
Consensus statement: summary of the Quebec Lung Cancer Network recommendations for prioritizing patients with thoracic cancers in the context of the COVID-19 pandemic.Curr Oncol. 2020 Jun;27(3):e313-e317. doi: 10.3747/co.27.6685. Epub 2020 Jun 1. Curr Oncol. 2020. PMID: 32669938 Free PMC article.
Cited by
-
Characterization of Metal-Bound Benzimidazole Derivatives, Effects on Tumor Cells of Lung Cancer.Materials (Basel). 2021 May 30;14(11):2958. doi: 10.3390/ma14112958. Materials (Basel). 2021. PMID: 34070886 Free PMC article.
-
International Association for the Study of Lung Cancer Study of the Impact of Coronavirus Disease 2019 on International Lung Cancer Clinical Trials.J Thorac Oncol. 2022 May;17(5):651-660. doi: 10.1016/j.jtho.2022.01.017. Epub 2022 Feb 17. J Thorac Oncol. 2022. PMID: 35183774 Free PMC article.
-
Imaging diagnosis of bronchogenic carcinoma (the forgotten disease) during times of COVID-19 pandemic: Current and future perspectives.World J Clin Oncol. 2021 Jun 24;12(6):437-457. doi: 10.5306/wjco.v12.i6.437. World J Clin Oncol. 2021. PMID: 34189068 Free PMC article. Review.
-
COVID-19 and Lung Cancer Survival: An Updated Systematic Review and Meta-Analysis.Cancers (Basel). 2022 Nov 21;14(22):5706. doi: 10.3390/cancers14225706. Cancers (Basel). 2022. PMID: 36428798 Free PMC article. Review.
-
COVID-19 and Lung Cancer: A Comprehensive Overview from Outbreak to Recovery.Biomedicines. 2022 Mar 26;10(4):776. doi: 10.3390/biomedicines10040776. Biomedicines. 2022. PMID: 35453526 Free PMC article. Review.
References
-
- Agenzia Italiana del Farmaco (AIFA). Available: https://www. aifa. gov. it/ documents/ 20142/ 871583/ Comunicato_ gestione_ studi_ clinici_ in_ emergenza_ COVID- 19_ EN_ 12. 03. 2020. pdf/ ee1f33e3- bb3e- 9ce9-2a93- b33e88eea94 (accessed 20 August 2020).
-
- European Medicines Agency (EMA). Available: https://www. ema. europa. eu/ en/ news/ guidance- sponsors- how- manage- clinical- trials- during- covid- 19- pandemic (accessed 20 August 2020).
-
- Food and Drug Administration (FDA). Available: https://www. fda. gov/ media/ 136238/ download (accessed 20 August 2020).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

