Fetal Rhesus Monkey First Trimester Zika Virus Infection Impacts Cortical Development in the Second and Third Trimesters
- PMID: 33341889
- PMCID: PMC8023859
- DOI: 10.1093/cercor/bhaa336
Fetal Rhesus Monkey First Trimester Zika Virus Infection Impacts Cortical Development in the Second and Third Trimesters
Abstract
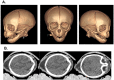
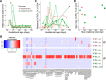
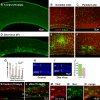
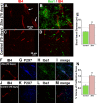
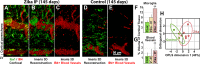
Zika virus is a teratogen similar to other neurotropic viruses, notably cytomegalovirus and rubella. The goal of these studies was to address the direct impact of Zika virus on fetal development by inoculating early gestation fetal rhesus monkeys using an ultrasound-guided approach (intraperitoneal vs. intraventricular). Growth and development were monitored across gestation, maternal samples collected, and fetal tissues obtained in the second trimester or near term. Although normal growth and anatomical development were observed, significant morphologic changes were noted in the cerebral cortex at 3-weeks post-Zika virus inoculation including massive alterations in the distribution, density, number, and morphology of microglial cells in proliferative regions of the fetal cerebral cortex; an altered distribution of Tbr2+ neural precursor cells; increased diameter and volume of blood vessels in the cortical proliferative zones; and a thinner cortical plate. At 3-months postinoculation, alterations in morphology, distribution, and density of microglial cells were also observed with an increase in blood vessel volume; and a thinner cortical plate. Only transient maternal viremia was observed but sustained maternal immune activation was detected. Overall, these studies suggest persistent changes in cortical structure result from early gestation Zika virus exposure with durable effects on microglial cells.
Keywords: Zika virus; fetus; microglia; monkey; vasculature.
© The Author(s) 2020. Published by Oxford University Press. All rights reserved. For permissions, please e-mail: journals.permission@oup.com.
Figures

References
-
- Andjelkovic AV, Nikolic B, Pachter JS, Zecevic N. 1998. Macrophages/microglial cells in human central nervous system during development: an immunohistochemical study. Brain Res. 814:13–25. - PubMed
-
- Arnò B, Galli F, Roostalu U, Aldeiri BM, Miyake T, Albertini A, Bragg L, Prehar S, McDermott JC, Cartwright EJ et al. 2014. Neural progenitor cells orchestrate microglia migration and positioning into the developing cortex. Nat Commun. 5:5611. - PubMed

