Efficacy and tolerability of preoperative chemoradiotherapy with S-1 alone for locally advanced rectal cancer
- PMID: 33341902
- PMCID: PMC7948831
- DOI: 10.1093/jrr/rraa117
Efficacy and tolerability of preoperative chemoradiotherapy with S-1 alone for locally advanced rectal cancer
Abstract
Preoperative chemoradiotherapy with capecitabine or 5-fluorouracil is a standard treatment for locally advanced rectal cancer (LARC). S-1, a prodrug of 5-fluorouracil, is a candidate for this chemoradiotherapy regimen in Japan; however, treatment outcomes after S-1 treatment alone are not clear. This study aimed to assess the efficacy and tolerability of preoperative chemoradiotherapy with S-1 alone for LARC. We retrospectively evaluated 54 LARC patients who underwent preoperative chemoradiotherapy with S-1 alone in our institution between 2005 and 2017. The clinical tumor stage was cT2-3 in 31 patients and cT4 in 23 patients, and lymph node metastases were clinically evident in 31 patients. S-1, at a dose of 80 mg/m2/day, was orally administered during radiotherapy. A total dose of 45-50.4 Gy was delivered in 25-28 fractions (median: 50.4 Gy). Surgical resections were scheduled 6-10 weeks after chemoradiotherapy completion. The 3- and 5-year overall survival rates were 92.4 and 72.8%, respectively, with a median follow-up time of 51 months. The 3- and 5-year local control rates were 96.2 and 85.9%, respectively. A pathological complete response was observed in 7 patients (13.0%) at the time of surgery. Ten patients (18.5%) had grade 3 acute toxicities and 5 patients (9.3%) had grade 3 late toxicities. No grade 4 or 5 toxicities were observed. Preoperative chemoradiotherapy with S-1 alone followed by total mesorectal excision resulted in a low incidence of toxicities and comparable clinical results. Therefore, S-1 alone can be a treatment option for preoperative chemoradiotherapy in LARC patients.
Keywords: S-1 alone; locally advanced rectal cancer; preoperative chemoradiotherapy.
© The Author(s) 2020. Published by Oxford University Press on behalf of The Japanese Radiation Research Society and Japanese Society for Radiation Oncology.
Figures
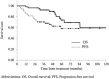
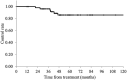
References
-
- Sauer R, Becker H, Hohenberger W et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731–40. - PubMed
-
- Sauer R, Liersch T, Merkel S et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: Results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol 2012;30:1926–33. - PubMed
-
- Bosset JF, Collette L, Calais G et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 2006;355:1114–23. - PubMed
-
- Gerard JP, Conroy T, Bonnetain F et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: Results of FFCD 9203. J Clin Oncol 2006;24:4620–5. - PubMed
-
- Bujko K, Nowacki MP, Nasierowska-Guttmejer A et al. Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg 2006;93:1215–23. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

