Comparison of four methods to measure haemoglobin concentrations in whole blood donors (COMPARE): A diagnostic accuracy study
- PMID: 33341984
- PMCID: PMC8048787
- DOI: 10.1111/tme.12750
Comparison of four methods to measure haemoglobin concentrations in whole blood donors (COMPARE): A diagnostic accuracy study
Abstract
Objective: To compare four haemoglobin measurement methods in whole blood donors.
Background: To safeguard donors, blood services measure haemoglobin concentration in advance of each donation. NHS Blood and Transplant's (NHSBT) customary method have been capillary gravimetry (copper sulphate), followed by venous spectrophotometry (HemoCue) for donors failing gravimetry. However, NHSBT's customary method results in 10% of donors being inappropriately bled (ie, with haemoglobin values below the regulatory threshold).
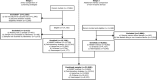
Methods: We compared the following four methods in 21 840 blood donors (aged ≥18 years) recruited from 10 NHSBT centres in England, with the Sysmex XN-2000 haematology analyser, the reference standard: (1) NHSBT's customary method; (2) "post donation" approach, that is, estimating current haemoglobin concentration from that measured by a haematology analyser at a donor's most recent prior donation; (3) "portable haemoglobinometry" (using capillary HemoCue); (4) non-invasive spectrometry (using MBR Haemospect or Orsense NMB200). We assessed sensitivity; specificity; proportion who would have been inappropriately bled, or rejected from donation ("deferred") incorrectly; and test preference.
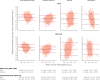
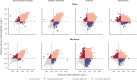
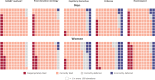
Results: Compared with the reference standard, the methods ranged in test sensitivity from 17.0% (MBR Haemospect) to 79.0% (portable haemoglobinometry) in men, and from 19.0% (MBR Haemospect) to 82.8% (portable haemoglobinometry) in women. For specificity, the methods ranged from 87.2% (MBR Haemospect) to 99.9% (NHSBT's customary method) in men, and from 74.1% (Orsense NMB200) to 99.8% (NHSBT's customary method) in women. The proportion of donors who would have been inappropriately bled ranged from 2.2% in men for portable haemoglobinometry to 18.9% in women for MBR Haemospect. The proportion of donors who would have been deferred incorrectly with haemoglobin concentration above the minimum threshold ranged from 0.1% in men for NHSBT's customary method to 20.3% in women for OrSense. Most donors preferred non-invasive spectrometry.
Conclusion: In the largest study reporting head-to-head comparisons of four methods to measure haemoglobin prior to blood donation, our results support replacement of NHSBT's customary method with portable haemoglobinometry.
Keywords: HemoCue; gravimetry; haemoglobin screening; inappropriate bleeding; inappropriate deferral; non-invasive haemoglobin measurement; whole blood donor.
© 2020 The Authors. Transfusion Medicine published by John Wiley & Sons Ltd on behalf of British Blood Transfusion Society.
Conflict of interest statement
John Danesh reports grants, personal fees and non‐financial support from Merck Sharp & Dohme (MSD), grants, personal fees and non‐financial support from Novartis, grants from Pfizer and grants from AstraZeneca outside the submitted work.
John Danesh serves on the International Cardiovascular and Metabolic Advisory Board for Novartis (since 2010); the Steering Committee of UK Biobank (since 2011); the MRC International Advisory Group (ING) member, London (since 2013); the MRC High Throughput Science Omics Panel Member, London (since 2013); the Scientific Advisory Committee for Sanofi (since 2013); the International Cardiovascular and Metabolism Research and Development Portfolio Committee for Novartis; and the AstraZeneca Genomics Advisory Board (2018).
Figures


References
-
- Cable RG. Hemoglobin determination in blood donors. Transfus Med Rev. 1995;9:131‐144. - PubMed
-
- Commission directive 2004/33/EC implementing directive 2002/98/EC of the European Parliament and of the council as regards certain technical requirements for blood and blood components. Official J Eur Union. 2004;256:32‐40.
-
- Zalpuri S, Romeijn B, Allara E, et al. Variations in hemoglobin measurement and eligibility criteria across blood donation services are associated with differing low‐hemoglobin deferral rates: a BEST collaborative study. Transfusion. 2020;60(3):544‐552. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MR/L003120/1/UK Medical Research Council
- RG/18/13/33946/BHF_/British Heart Foundation/United Kingdom
- RG/13/13/30194/BHF_/British Heart Foundation/United Kingdom
- NHSBT
- NIHR BioResource
- NIHR BTRU-2014-10024/NIHR Blood and Transplant Research Unit (BTRU) in Donor Health and Genomics
- NIHR [Cambridge Biomedical Research Centre at the Cambridge University Hospitals NHS Foundation Trust]
- Health Data Research UK
- Engineering and Physical Sciences Research Council
- WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Medical

