Guidelines for the design and conduct of human clinical trials on ingestion-time differences - chronopharmacology and chronotherapy - of hypertension medications
- PMID: 33342316
- PMCID: PMC8112296
- DOI: 10.1080/07420528.2020.1850468
Guidelines for the design and conduct of human clinical trials on ingestion-time differences - chronopharmacology and chronotherapy - of hypertension medications
Abstract
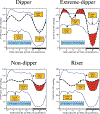
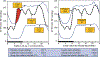
Current hypertension guidelines fail to provide a recommendation on when-to-treat, thus disregarding relevant circadian rhythms that regulate blood pressure (BP) level and 24 h patterning and medication pharmacokinetics and pharmacodynamics. The ideal purpose of ingestion-time (chronopharmacology, i.e. biological rhythm-dependent effects on the kinetics and dynamics of medications, and chronotherapy, i.e. the timing of pharmaceutical and other treatments to optimize efficacy and safety) trials should be to explore the potential impact of endogenous circadian rhythms on the effects of medications. Such investigations and outcome trials mandate adherence to the basic standards of human chronobiology research. In-depth review of the more than 150 human hypertension pharmacology and therapeutic trials published since 1974 that address the differential impact of upon-waking/morning versus at-bedtime/evening schedule of treatment reveals diverse protocols of sometimes suboptimal or defective design and conduct. Many have been "time-of-day," i.e. morning versus evening, rather than circadian-time-based, and some relied on wake-time office BP rather than around-the-clock ambulatory BP measurements (ABPM). Additionally, most past studies have been of too small sample size and thus statistically underpowered. As of yet, there has been no consensual agreement on the proper design, methods and conduct of such trials. This Position Statement recommends ingestion-time hypertension trials to follow minimum guidelines: (i) Recruitment of participants should be restricted to hypertensive individuals diagnosed according to ABPM diagnostic thresholds and of a comparable activity/sleep routine. (ii) Tested treatment-times should be selected according to internal biological time, expressed by the awakening and bed times of the sleep/wake cycle. (iii) ABPM should be the primary or sole method of BP assessment. (iv) The minimum-required features for analysis of the ABPM-determined 24 h BP pattern ought to be the asleep (not "nighttime") BP mean and sleep-time relative BP decline, calculated in reference to the activity/rest cycle per individual. (v) ABPM-obtained BP means should be derived by the so-called adjusted calculation procedure, not by inaccurate arithmetic averages. (vi) ABPM should be performed with validated and calibrated devices at least hourly throughout two or more consecutive 24 h periods (48 h in total) to achieve the highest reproducibility of mean wake-time, sleep-time and 48 h BP values plus the reliable classification of dipping status. (vii) Calculation of minimum required sample size in adherence with proper statistical methods must be provided. (viii) Hypertension chronopharmacology and chronotherapy trials should preferably be randomized double-blind, randomized open-label with blinded-endpoint, or crossover in design, the latter with sufficient washout period between tested treatment-time regimens.
Keywords: Ambulatory blood pressure monitoring; blood pressure dipping; hypertension chronotherapy; hypertension medications; sleep-time blood pressure; trial design recommendations and guidelines.
Conflict of interest statement
Disclosure statement
The authors report no conflicts of interest.
Figures



Similar articles
-
Systematic review and quality evaluation of published human ingestion-time trials of blood pressure-lowering medications and their combinations.Chronobiol Int. 2021 Oct;38(10):1460-1476. doi: 10.1080/07420528.2021.1931280. Epub 2021 Jun 9. Chronobiol Int. 2021. PMID: 34107831
-
Elevated asleep blood pressure and non-dipper 24h patterning best predict risk for heart failure that can be averted by bedtime hypertension chronotherapy: A review of the published literature.Chronobiol Int. 2023 Jan;40(1):63-82. doi: 10.1080/07420528.2021.1939367. Epub 2021 Jun 30. Chronobiol Int. 2023. PMID: 34190016 Review.
-
Sleep-time ambulatory blood pressure as a prognostic marker of vascular and other risks and therapeutic target for prevention by hypertension chronotherapy: Rationale and design of the Hygia Project.Chronobiol Int. 2016;33(7):906-36. doi: 10.1080/07420528.2016.1181078. Epub 2016 May 24. Chronobiol Int. 2016. PMID: 27221952
-
Extent of asleep blood pressure reduction by hypertension medications is ingestion-time dependent: Systematic review and meta-analysis of published human trials.Sleep Med Rev. 2021 Oct;59:101454. doi: 10.1016/j.smrv.2021.101454. Epub 2021 Jan 23. Sleep Med Rev. 2021. PMID: 33571840
-
Effects of time-of-day of hypertension treatment on ambulatory blood pressure and clinical characteristics of patients with type 2 diabetes.Chronobiol Int. 2013 Mar;30(1-2):116-31. doi: 10.3109/07420528.2012.702587. Epub 2012 Oct 25. Chronobiol Int. 2013. PMID: 23181613
Cited by
-
Association of ambulatory blood pressure with coronary microvascular and cardiac dysfunction in asymptomatic type 2 diabetes.Cardiovasc Diabetol. 2022 May 28;21(1):85. doi: 10.1186/s12933-022-01528-2. Cardiovasc Diabetol. 2022. PMID: 35643571 Free PMC article.
-
Pharmacogenomics and circadian rhythms as mediators of cardiovascular drug-drug interactions.Curr Res Pharmacol Drug Discov. 2021 May 6;2:100025. doi: 10.1016/j.crphar.2021.100025. eCollection 2021. Curr Res Pharmacol Drug Discov. 2021. PMID: 34909660 Free PMC article. Review.
-
The intersection of circadian rhythms and the blood-brain barrier with drug efficacy and delivery in neurological disorders.Adv Drug Deliv Rev. 2025 Sep;224:115645. doi: 10.1016/j.addr.2025.115645. Epub 2025 Jul 2. Adv Drug Deliv Rev. 2025. PMID: 40614866 Review.
References
-
- Angeli A, Gatti G, Masera R. 1992. Chronobiology of the hypothalamic-pituitary-adrenal and renin-angiotensin-aldosterone systems. In: Touitou Y, Haus E, editors. Biologic rhythms in clinical and laboratory medicine. Berlin: Springer-Verlag; p. 292–314.
-
- Ayala DE, Moyá A, Crespo JJ, Castiñeira C, Domínguez-Sardiña M, Gomara S, Sineiro E, Mojón A, Fontao MJ, Hermida RC. 2013b. Circadian pattern of ambulatory blood presure in hypertensive patients with and without type 2 diabetes. Chronobiol Int. 30:99–115. doi:10.3109/07420528.2012.701489 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
