IPSE, a parasite-derived, host immunomodulatory infiltrin protein, alleviates resiniferatoxin-induced bladder pain
- PMID: 33342372
- PMCID: PMC7756320
- DOI: 10.1177/1744806920970099
IPSE, a parasite-derived, host immunomodulatory infiltrin protein, alleviates resiniferatoxin-induced bladder pain
Abstract
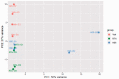
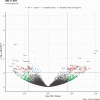
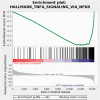
The transient receptor potential cation channel subfamily V member 1 (TRPV1) receptor is an important mediator of nociception and its expression is enriched in nociceptive neurons. TRPV1 signaling has been implicated in bladder pain and is a potential analgesic target. Resiniferatoxin is the most potent known agonist of TRPV1. Acute exposure of the rat bladder to resiniferatoxin has been demonstrated to result in pain-related freezing and licking behaviors that are alleviated by virally encoded IL-4. The interleukin-4-inducing principle of Schistosoma mansoni eggs (IPSE) is a powerful inducer of IL-4 secretion, and is also known to alter host cell transcription through a nuclear localization sequence-based mechanism. We previously reported that IPSE ameliorates ifosfamide-induced bladder pain in an IL-4- and nuclear localization sequence-dependent manner. We hypothesized that pre-administration of IPSE to resiniferatoxin-challenged mice would dampen pain-related behaviors. IPSE indeed lessened resiniferatoxin-triggered freezing behaviors in mice. This was a nuclear localization sequence-dependent phenomenon, since administration of a nuclear localization sequence mutant version of IPSE abrogated IPSE's analgesic effect. In contrast, IPSE's analgesic effect did not seem IL-4-dependent, since use of anti-IL-4 antibody in mice given both IPSE and resiniferatoxin did not significantly affect freezing behaviors. RNA-Seq analysis of resiniferatoxin- and IPSE-exposed bladders revealed differential expression of TNF/NF-κb-related signaling pathway genes. In vitro testing of IPSE uptake by urothelial cells and TRPV1-expressing neuronal cells showed uptake by both cell types. Thus, IPSE's nuclear localization sequence-dependent therapeutic effects on TRPV1-mediated bladder pain may act on TRPV1-expressing neurons and/or may rely upon urothelial mechanisms.
Keywords: Bladder; analgesic; immune modulation; pain; parasite; schistosoma.
Conflict of interest statement
Figures




References
-
- Schramm G, Gronow A, Knobloch J, Wippersteg V, Grevelding CG, Galle J, Fuller H, Stanley RG, Chiodini PL, Haas H, Doenhoff MJ. IPSE/alpha-1: a major immunogenic component secreted from Schistosoma mansoni eggs. Mol Biochem Parasitol 2006; 147: 9–19. - PubMed
-
- Kaur I, Schramm G, Everts B, Scholzen T, Kindle KB, Beetz C, Montiel-Duarte C, Blindow S, Jones AT, Haas H, Stolnik S, Heery DM, Falcone FH. Interleukin-4-inducing principle from Schistosoma mansoni eggs contains a functional C-terminal nuclear localization signal necessary for nuclear translocation in mammalian cells but not for its uptake. Infect Immun 2011; 79: 1779–1788. - PMC - PubMed
-
- Mbanefo EC, Le L, Zee R, Banskota N, Ishida K, Pennington LF, Odegaard JI, Jardetzky TS, Alouffi A, Falcone FH, Hsieh MH. IPSE, a urogenital parasite-derived immunomodulatory protein, ameliorates ifosfamide-induced hemorrhagic cystitis through downregulation of pro-inflammatory pathways. Sci Rep 2019; 9: 1–13. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

