Plasma and Fecal Metabolite Profiles in Autism Spectrum Disorder
- PMID: 33342544
- PMCID: PMC7867605
- DOI: 10.1016/j.biopsych.2020.09.025
Plasma and Fecal Metabolite Profiles in Autism Spectrum Disorder
Abstract
Background: Autism spectrum disorder (ASD) is a neurodevelopmental condition with hallmark behavioral manifestations including impaired social communication and restricted repetitive behavior. In addition, many affected individuals display metabolic imbalances, immune dysregulation, gastrointestinal dysfunction, and altered gut microbiome compositions.
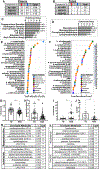
Methods: We sought to better understand nonbehavioral features of ASD by determining molecular signatures in peripheral tissues through mass spectrometry methods (ultrahigh performance liquid chromatography-tandem mass spectrometry) with broad panels of identified metabolites. Herein, we compared the global metabolome of 231 plasma and 97 fecal samples from a large cohort of children with ASD and typically developing control children.
Results: Differences in amino acid, lipid, and xenobiotic metabolism distinguished ASD and typically developing samples. Our results implicated oxidative stress and mitochondrial dysfunction, hormone level elevations, lipid profile changes, and altered levels of phenolic microbial metabolites. We also revealed correlations between specific metabolite profiles and clinical behavior scores. Furthermore, a summary of metabolites modestly associated with gastrointestinal dysfunction in ASD is provided, and a pilot study of metabolites that can be transferred via fecal microbial transplant into mice is identified.
Conclusions: These findings support a connection between metabolism, gastrointestinal physiology, and complex behavioral traits and may advance discovery and development of molecular biomarkers for ASD.
Keywords: ASD; Autism spectrum disorder; Fecal metabolites; Metabolomics; Mitochondrial dysfunction; Phenolic metabolites; Plasma metabolites; Steroid hormones.
Copyright © 2020 Society of Biological Psychiatry. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests
A.S.C., D.H.D. and S.K.M. have financial interest in Axial Biotherapeutics. A.F. has financial interest in Alba Therapeutics. G.M.P. and M.C.C. are employed by Axial Biotherapeutics. All other authors report no biomedical financial interests or potential conflicts of interest.
Figures





References
-
- Doernberg E, Hollander E, Neurodevelopmental Disorders (ASD and ADHD): DSM-5, ICD-10, and ICD-11. CNS Spectr. 21, 295–299 (2016). - PubMed
-
- Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z, Kurzius-Spencer M, Zahorodny W, Robinson Rosenberg C, White T, Durkin MS, Imm P, Nikolaou L, Yeargin-Allsopp M, Lee L-C, Harrington R, Lopez M, Fitzgerald RT, Hewitt A, Pettygrove S, Constantino JN, Vehorn A, Shenouda J, Hall-Lande J, Van Naarden Braun K, Dowling NF, Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years — Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014. MMWR Surveill. Summ 67, 1–23 (2018). - PMC - PubMed
-
- Virués-Ortega J, Applied behavior analytic intervention for autism in early childhood: meta-analysis, meta-regression and dose-response meta-analysis of multiple outcomes. Clin. Psychol. Rev 30, 387–399 (2010). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

