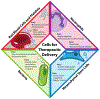
Biological Cells as Therapeutic Delivery Vehicles
- PMID: 33342562
- PMCID: PMC7856260
- DOI: 10.1016/j.tips.2020.11.008
Biological Cells as Therapeutic Delivery Vehicles
Abstract
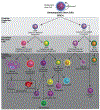
One of the significant challenges remaining in the field of drug delivery is insufficient targeting of diseased tissues or cells. While efforts to perform targeted drug delivery by engineered nanoparticles have shown some success, there are underlying targeting, toxicity, and immunogenicity challenges. By contrast, live cells usually have innate targeting mechanisms, and can be used as drug-delivery vehicles to increase the efficiency with which a drug accumulates to act on the intended tissue. In some cases, when no native cell types exhibit the desired therapeutic phenotype, preferred outcomes can be achieved by genetically modifying and reprogramming cells with gene circuits. This review highlights recent advances in the use of cells to deliver therapeutics. Specifically, we discuss how red blood cells (RBCs), platelets, neutrophils, mesenchymal stem cells (MSCs), and bacteria have been utilized to advance drug delivery.
Keywords: drug delivery; synthetic biology; therapeutic cells.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Disclaimer Statement
There are no conflicts to declare.
Figures

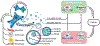
References
-
- Park K (2019) The beginning of the end of the nanomedicine hype. J Control Release 305, 221–222. - PubMed
-
- Wilhelm S et al. (2016) Analysis of nanoparticle delivery to tumors. Nature Reviews Materials 1.
-
- Fliervoet LAL and Mastrobattista E (2016) Drug delivery with living cells. Adv Drug Deliv Rev 106 (Pt A), 63–72. - PubMed
-
- Agrahari V et al. (2017) Next generation drug delivery: circulatory cells-mediated nanotherapeutic approaches. Expert Opin Drug Deliv 14 (3), 285–289. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

