Biomarkers: Opportunities and Challenges for Drug Development in the Current Regulatory Landscape
- PMID: 33343195
- PMCID: PMC7727038
- DOI: 10.1177/1177271920974652
Biomarkers: Opportunities and Challenges for Drug Development in the Current Regulatory Landscape
Abstract
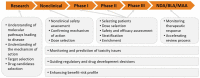
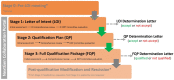
Biomarkers are widely used at every stage of drug discovery and development. Utilisation of biomarkers has a potential to make drug discovery, development and approval processes more efficient. An overview of the current global regulatory landscape is presented in this article with particular emphasis on the validation and qualification of biomarkers, as well as legal framework for companion diagnostics. Furthermore, this article shows how the number of approved drugs with at least 1 biomarker used during development (biomarker acceptance) is affected by the recent advances in the biomarker regulations. More than half of analysed approvals were supported by biomarker data and there has been a slight increase in acceptance of biomarkers in recent years, even though the growth is not continuous. For certain pharmacotherapeutic groups, approvals with biomarkers are more common than without. Examples include immunosuppressants, immunostimulants, drugs used in diabetes, antithrombotic drugs, antineoplastic agents and antivirals. As a conclusion, potential benefits, challenges and opportunities of using biomarkers in drug discovery and development in the current regulatory landscape are summarised and discussed.
Keywords: Biomarker; EMA; FDA; companion diagnostics; drug approval; drug development; qualification; regulatory landscape.
© The Author(s) 2020.
Conflict of interest statement
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Figures




References
-
- Institute of Medicine (US) Forum on Drug Discovery Development and Translation. 1. Introduction. In: Steve O, Sally R, Robert G, et al., eds. Accelerating the Development of Biomarkers for Drug Safety: Workshop Summary. National Academies Press (US); 2009: 1-6. - PubMed
-
- Seimetz D. The key to successful drug approval: an effective regulatory strategy. In: Becker J, Villinger TR, eds. Life Science Venturing. Springer Fachmedien Wiesbaden GmbH; 2017: 139–147.
-
- EMA. Glossary: biomarker. 2020. Accessed February 23, 2020 https://www.ema.europa.eu/en/glossary/biomarker
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

