Repositioning of Immunomodulators: A Ray of Hope for Alzheimer's Disease?
- PMID: 33343293
- PMCID: PMC7746859
- DOI: 10.3389/fnins.2020.614643
Repositioning of Immunomodulators: A Ray of Hope for Alzheimer's Disease?
Abstract
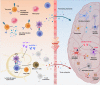
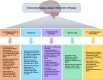
Alzheimer's disease (AD) is the most common age-related neurodegenerative disorder characterized by cognitive decline and by the presence of amyloid β plaques and neurofibrillary tangles in the brain. Despite recent advances in understanding its pathophysiological mechanisms, to date, there are no disease-modifying therapeutic options, to slow or halt the evolution of neurodegenerative processes in AD. Current pharmacological treatments only transiently mitigate the severity of symptoms, with modest or null overall improvement. Emerging evidence supports the concept that AD is affected by the impaired ability of the immune system to restrain the brain's pathology. Deep understanding of the relationship between the nervous and the immune system may provide a novel arena to develop effective and safe drugs for AD treatment. Considering the crucial role of inflammatory/immune pathways in AD, here we discuss the current status of the immuno-oncological, immunomodulatory and anti-TNF-α drugs which are being used in preclinical studies or in ongoing clinical trials by means of the drug-repositioning approach.
Keywords: clinical trial; disease-modifying therapy; drug repurposing; immune response; neuroinflammation.
Copyright © 2020 Munafò, Burgaletto, Di Benedetto, Di Mauro, Di Mauro, Bernardini and Cantarella.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The handling editor declared a past co-authorship with several of the authors GD, CB, RB, and GC.
Figures

References
-
- Arnon R., Aharoni R. (2019). Glatiramer acetate: from bench to bed and back. Isr. Med. Assoc. J. 21 151–157. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

