New Insights Into Drug Discovery Targeting Tau Protein
- PMID: 33343298
- PMCID: PMC7744460
- DOI: 10.3389/fnmol.2020.590896
New Insights Into Drug Discovery Targeting Tau Protein
Abstract
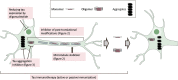
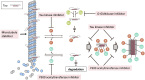
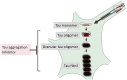
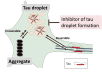
Microtubule-associated protein tau is characterized by the fact that it is an intrinsically disordered protein due to its lack of a stable conformation and high flexibility. Intracellular inclusions of fibrillar forms of tau with a β-sheet structure accumulate in the brain of patients with Alzheimer's disease and other tauopathies. Accordingly, detachment of tau from microtubules and transition of tau from a disordered state to an abnormally aggregated state are essential events preceding the onset of tau-related diseases. Many reports have shown that this transition is caused by post-translational modifications, including hyperphosphorylation and acetylation. The misfolded tau is self-assembled and forms a tau oligomer before the appearance of tau inclusions. Animal and pathological studies using human samples have demonstrated that tau oligomer formation contributes to neuronal loss. During the progression of tauopathies, tau seeds are released from cells and incorporated into other cells, leading to the propagation of pathological tau aggregation. Accumulating evidence suggests several potential approaches for blocking tau-mediated toxicity: (1) direct inhibition of pathological tau aggregation and (2) inhibition of tau post-translational modifications that occur prior to pathological tau aggregation, (3) inhibition of tau propagation and (4) stabilization of microtubules. In addition to traditional low-molecular-weight compounds, newer drug discovery approaches such as the development of medium-molecular-weight drugs (peptide- or oligonucleotide-based drugs) and high-molecular-weight drugs (antibody-based drugs) provide alternative pathways to preventing the formation of abnormal tau. Of particular interest are recent studies suggesting that tau droplet formation by liquid-liquid phase separation may be the initial step in aberrant tau aggregation, as well results that implicate roles for tau in dendritic and nuclear functions. Here, we review the mechanisms through which drugs can target tau and consider recent clinical trials for the treatment of tauopathies. In addition, we discuss the utility of these newer strategies and propose future directions for research on tau-targeted therapeutics.
Keywords: aggregation; immunotherapy; inflammation; liquid-liquid phase separation; microtubule stabilizer; oligonucleotide therapy; post-translational modifications; tau protein.
Copyright © 2020 Soeda and Takashima.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

