Contact-Chemosensory Evolution Underlying Reproductive Isolation in Drosophila Species
- PMID: 33343311
- PMCID: PMC7746553
- DOI: 10.3389/fnbeh.2020.597428
Contact-Chemosensory Evolution Underlying Reproductive Isolation in Drosophila Species
Abstract
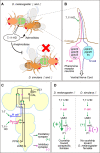
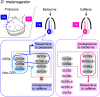
The main theme of the review is how changes in pheromone biochemistry and the sensory circuits underlying pheromone detection contribute to mate choice and reproductive isolation. The review focuses primarily on gustatory and non-volatile signals in Drosophila. Premating isolation is prevalent among closely related species. In Drosophila, preference for conspecifics against other species in mate choice underlies premating isolation, and such preference relies on contact chemosensory communications between a female and male along with other biological factors. For example, although D. simulans and D. melanogaster are sibling species that yield hybrids, their premating isolation is maintained primarily by the contrasting effects of 7,11-heptacosadiene (7,11-HD), a predominant female pheromone in D. melanogaster, on males of the two species: it attracts D. melanogaster males and repels D. simulans males. The contrasting preference for 7,11-HD in males of these two species is mainly ascribed to opposite effects of 7,11-HD on neural activities in the courtship decision-making neurons in the male brain: 7,11-HD provokes both excitatory and inhibitory inputs in these neurons and differences in the balance between the two counteracting inputs result in the contrasting preference for 7,11-HD, i.e., attraction in D. melanogaster and repulsion in D. simulans. Introduction of two double bonds is a key step in 7,11-HD biosynthesis and is mediated by the desaturase desatF, which is active in D. melanogaster females but transcriptionally inactivated in D. simulans females. Thus, 7,11-HD biosynthesis diversified in females and 7,11-HD perception diversified in males, yet it remains elusive how concordance of the changes in the two sexes was attained in evolution.
Keywords: central integration; doublesex; fruitless; gustatory receptors; hybrids; hydrocarbon metabolism; pheromones; premating isolation.
Copyright © 2020 Sato and Yamamoto.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

