Gastrin Attenuates Renal Ischemia/Reperfusion Injury by a PI3K/Akt/Bad-Mediated Anti-apoptosis Signaling
- PMID: 33343341
- PMCID: PMC7740972
- DOI: 10.3389/fphar.2020.540479
Gastrin Attenuates Renal Ischemia/Reperfusion Injury by a PI3K/Akt/Bad-Mediated Anti-apoptosis Signaling
Abstract
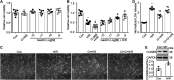
Ischemic/reperfusion (I/R) injury is the primary cause of acute kidney injury (AKI). Gastrin, a gastrointestinal hormone, is involved in the regulation of kidney function of sodium excretion. However, whether gastrin has an effect on kidney I/R injury is unknown. Here we show that cholecystokinin B receptor (CCKBR), the gastrin receptor, was significantly up-regulated in I/R-injured mouse kidneys. While pre-administration of gastrin ameliorated I/R-induced renal pathological damage, as reflected by the levels of serum creatinine and blood urea nitrogen, hematoxylin and eosin staining and periodic acid-Schiff staining. The protective effect could be ascribed to the reduced apoptosis for gastrin reduced tubular cell apoptosis both in vivo and in vitro. In vitro studies also showed gastrin preserved the viability of hypoxia/reoxygenation (H/R)-treated human kidney 2 (HK-2) cells and reduced the lactate dehydrogenase release, which were blocked by CI-988, a specific CCKBR antagonist. Mechanistically, the PI3K/Akt/Bad pathway participates in the pathological process, because gastrin treatment increased phosphorylation of PI3K, Akt and Bad. While in the presence of wortmannin (1 μM), a PI3K inhibitor, the gastrin-induced phosphorylation of Akt after H/R treatment was blocked. Additionally, wortmannin and Akt inhibitor VIII blocked the protective effect of gastrin on viability of HK-2 cells subjected to H/R treatment. These studies reveals that gastrin attenuates kidney I/R injury via a PI3K/Akt/Bad-mediated anti-apoptosis signaling. Thus, gastrin can be considered as a promising drug candidate to prevent AKI.
Keywords: Akt; CCKBR; acute kidney injury; apoptosis; gastrin; ischemia-reperfusion injury.
Copyright © 2020 Liu, Chen, Wang, Zhang, Duan, Wang and Zeng.
Figures






References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

