Efficacy and Safety of CAR-Modified T Cell Therapy in Patients with Relapsed or Refractory Multiple Myeloma: A Meta-Analysis of Prospective Clinical Trials
- PMID: 33343342
- PMCID: PMC7744881
- DOI: 10.3389/fphar.2020.544754
Efficacy and Safety of CAR-Modified T Cell Therapy in Patients with Relapsed or Refractory Multiple Myeloma: A Meta-Analysis of Prospective Clinical Trials
Abstract
Background: In recent years, chimeric antigen receptor-modified T (CAR-T) cell therapy for B-cell leukemia and lymphoma has shown high clinical efficacy. Similar CAR-T clinical trials have also been carried out in patients with refractory/relapsed multiple myeloma (RRMM). However, no systematic review has evaluated the efficacy and safety of CAR-T cell therapy in RRMM. The purpose of this study was to fill this literature gap. Methods: Eligible studies were searched in PUBMED, EMBASE, the Cochrane Central Register of Controlled Trials (CENTRAL), CNKI, and WanFang from data inception to December 2019. For efficacy assessment, the overall response rate (ORR), minimal residual disease (MRD) negativity rate, strict complete response (sCR), complete response (CR), very good partial response (VGPR), and partial response (PR) were calculated. The incidence of any grade cytokine release syndrome (CRS) and grade ≥3 adverse events (AEs) were calculated for safety analysis. The effect estimates were then pooled using an inverse variance method. Results: Overall, 27 studies involving 497 patients were included in this meta-analysis. The pooled ORR and MRD negativity rate were 89% (95% Cl: 83-94%) and 81% (95% Cl: 67-91%), respectively. The pooled sCR, CR, VGPR, and PR were 14% (95% Cl: 5-27%), 13% (95% Cl: 4-26%), 23% (95% Cl: 14-33%), and 15% (95% Cl: 10-21%), respectively. Subgroup analyses of ORR by age, proportion of previous autologous stem cell transplantation (ASCT), and target selection of CAR-T cells revealed that age ≤ 55 years (≤55 years vs. > 55 years, p = 0.0081), prior ASCT ≤70% (≤70% vs. > 70%, p = 0.035), and bispecific CAR-T cells (dual B-cell maturation antigen (BCMA)/BCMA + CD19 vs specific BCMA, p = 0.0329) associated with higher ORR in patients. Subgroup analyses of remission depth by target selection suggested that more patients achieved a better response than VGPR with dual BCMA/BCMA + CD19 CAR-T cells compared to specific BCMA targeting (p = 0.0061). In terms of safety, the pooled incidence of any grade and grade ≥ 3 CRS was 76% (95% CL: 63-87%) and 11% (95% CL: 6-17%). The most common grade ≥ 3 AEs were hematologic toxic effects. Conclusion: In heavily treated patients, CAR-T therapy associates with promising responses and tolerable AEs, as well as CRS in RRMM. However, additional information regarding the durability of CAR-T cell therapy, as well as further randomized controlled trials, is needed.
Keywords: cancer immunotherapy; chimeric antigen receptor; efficacy; meta-analysis; multiple myeloma; safety.
Copyright © 2020 Xiang, He, Ou, Wang and Wu.
Conflict of interest statement
The authors state that the research was performed in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
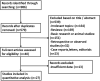



References
-
- Bellucci R., Alyea E. P., Chiaretti S., Wu C. J., Zorn E., Weller E., et al. (2005). Graft-versus-tumor response in patients with multiple myeloma is associated with antibody response to BCMA, a plasma-cell membrane receptor. Blood 105 (10), 3945–3950. 10.1182/blood-2004-11-4463 - DOI - PMC - PubMed
-
- Berdeja J. G., Alsina M., Shah N. D., Siegel D. S., Jagannath S., Madduri D., et al. (2019). Updated results from an ongoing phase 1 clinical study of bb21217 anti-Bcma CAR T cell therapy. Blood 134 (1), 927 10.1182/blood-2019-126660 - DOI
-
- Brudno J. N., Maric I., Hartman S. D., Rose J. J., Wang M., Lam N., et al. (2018). T cells genetically modified to express an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of poor-prognosis relapsed multiple myeloma. J. Clin. Oncol. 36(22), 2267–2280. 10.1200/jco.2018.77.8084 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

