Biosensor and Lab-on-a-chip Biomarker-identifying Technologies for Oral and Periodontal Diseases
- PMID: 33343358
- PMCID: PMC7748088
- DOI: 10.3389/fphar.2020.588480
Biosensor and Lab-on-a-chip Biomarker-identifying Technologies for Oral and Periodontal Diseases
Abstract
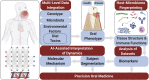
Periodontitis is a complex multifactorial disease that can lead to destruction of tooth supporting tissues and subsequent tooth loss. The most recent global burden of disease studies highlight that severe periodontitis is one of the most prevalent chronic inflammatory conditions affecting humans. Periodontitis risk is attributed to genetics, host-microbiome and environmental factors. Empirical diagnostic and prognostic systems have yet to be validated in the field of periodontics. Early diagnosis and intervention prevents periodontitis progression in most patients. Increased susceptibility and suboptimal control of modifiable risk factors can result in poor response to therapy, and relapse. The chronic immune-inflammatory response to microbial biofilms at the tooth or dental implant surface is associated with systemic conditions such as cardiovascular disease, diabetes or gastrointestinal diseases. Oral fluid-based biomarkers have demonstrated easy accessibility and potential as diagnostics for oral and systemic diseases, including the identification of SARS-CoV-2 in saliva. Advances in biotechnology have led to innovations in lab-on-a-chip and biosensors to interface with oral-based biomarker assessment. This review highlights new developments in oral biomarker discovery and their validation for clinical application to advance precision oral medicine through improved diagnosis, prognosis and patient stratification. Their potential to improve clinical outcomes of periodontitis and associated chronic conditions will benefit the dental and overall public health.
Keywords: biomarkers; biotechnology; patient stratification; periodontal diseases/periodontitis; precision medicine; saliva.
Copyright © 2020 Steigmann, Maekawa, Sima, Travan, Wang and Giannobile.
Figures



Similar articles
-
Periodontal Disease as a Risk Factor for Rheumatoid Arthritis: A Systematic Review.JBI Libr Syst Rev. 2012;10(42 Suppl):1-12. doi: 10.11124/jbisrir-2012-288. JBI Libr Syst Rev. 2012. PMID: 27820156
-
The potential impact of salivary peptides in periodontitis.Crit Rev Clin Lab Sci. 2021 Nov;58(7):479-492. doi: 10.1080/10408363.2021.1907298. Epub 2021 Apr 13. Crit Rev Clin Lab Sci. 2021. PMID: 33849374 Review.
-
Bridging oral and systemic health: exploring pathogenesis, biomarkers, and diagnostic innovations in periodontal disease.Infection. 2025 May 26. doi: 10.1007/s15010-025-02568-y. Online ahead of print. Infection. 2025. PMID: 40418274 Review.
-
Identification of pathogen and host-response markers correlated with periodontal disease.J Periodontol. 2009 Mar;80(3):436-46. doi: 10.1902/jop.2009.080480. J Periodontol. 2009. PMID: 19254128 Free PMC article. Clinical Trial.
-
Periodontal manifestations of systemic diseases and developmental and acquired conditions: Consensus report of workgroup 3 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions.J Clin Periodontol. 2018 Jun;45 Suppl 20:S219-S229. doi: 10.1111/jcpe.12951. J Clin Periodontol. 2018. PMID: 29926500
Cited by
-
Potential Impact of Prosthetic Biomaterials on the Periodontium: A Comprehensive Review.Molecules. 2023 Jan 20;28(3):1075. doi: 10.3390/molecules28031075. Molecules. 2023. PMID: 36770741 Free PMC article. Review.
-
Molecular Screening and Analysis Reveal Novel Oral Site-Specific Locations for the Cariogenic Pathogen Scardovia wiggsiae.Dent J (Basel). 2021 Jun 17;9(6):73. doi: 10.3390/dj9060073. Dent J (Basel). 2021. PMID: 34204219 Free PMC article.
-
Nanotechnology and periodontics.J Periodontal Implant Sci. 2023 Aug;53(4):245-247. doi: 10.5051/jpis.235304edi01. J Periodontal Implant Sci. 2023. PMID: 37635654 Free PMC article. No abstract available.
-
Green Synthesis of Bacopa monnieri-Mediated Magnesium Oxide Nanoparticles and Analysis of Their Antimicrobial, Antioxidant, and Cytotoxic Properties.Cureus. 2024 Jan 22;16(1):e52701. doi: 10.7759/cureus.52701. eCollection 2024 Jan. Cureus. 2024. PMID: 38384608 Free PMC article.
-
Molecular biomarker research in periodontology: A roadmap for translation of science to clinical assay validation.J Clin Periodontol. 2022 Jun;49(6):556-561. doi: 10.1111/jcpe.13617. Epub 2022 Apr 3. J Clin Periodontol. 2022. PMID: 35322451 Free PMC article.
References
-
- Afacan B., Keleş Yücel Z. P., Paşali Ç., Atmaca İlhan H., Köse T., Emingil G. (2020). Effect of non‐surgical periodontal treatment on gingival crevicular fluid hypoxia inducible factor‐1 alpha, vascular endothelial growth factor and tumor necrosis factor‐alpha levels in generalized aggressive periodontitis patients. J. Periodontol. 1–8. 10.1002/JPER.19-0521 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

