Regulating Gut Microbiome: Therapeutic Strategy for Rheumatoid Arthritis During Pregnancy and Lactation
- PMID: 33343364
- PMCID: PMC7748111
- DOI: 10.3389/fphar.2020.594042
Regulating Gut Microbiome: Therapeutic Strategy for Rheumatoid Arthritis During Pregnancy and Lactation
Abstract
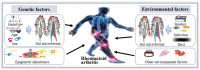
Rheumatoid arthritis (RA) is an autoimmune disease characterized by synovial inflammation and bone destruction. Microbial infection is considered to be the most important inducement of RA. The pregnancy planning of women in childbearing age is seriously affected by the disease activity of RA. Gut microbiome, related to immunity and inflammatory response of the host. At present, emerging evidence suggested there are significant differences in the diversity and abundance of gut microbiome during pregnancy and lactation, which may be associated with the fluctuation of RA disease activity. Based on these research foundations, we pioneer the idea of regulating gut microbiome for the treatment of RA during pregnancy and lactation. In this review, we mainly introduce the potential treatment strategies for controlling the disease activity of RA based on gut microbiome during pregnancy and lactation. Besides, we also briefly generalize the effects of conventional anti-rheumatic drugs on gut microbiome, the effects of metabolic changes during pregnancy on gut microbiome, alteration of gut microbiome during pregnancy and lactation, and the effects of anti-rheumatic drugs commonly used during pregnancy and lactation on gut microbiome. These will provide a clear knowledge framework for researchers in immune-related diseases during pregnancy. Regulating gut microbiome may be a potential and effective treatment to control the disease activity of RA during pregnancy and lactation.
Keywords: gut microbiome; lactation; pregnancy; rheumatoid arthritis; treatment.
Copyright © 2020 Yao, Cai, Fei, Ren, Wang, Luan, Chen and Zheng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Angelakis E., Million M., Kankoe S., Lagier J. C., Armougom F., Giorgi R., et al. (2014). Abnormal weight gain and gut microbiota modifications are side effects of long-term doxycycline and hydroxychloroquine treatment. Antimicrob. Agents Chemother. 58 (6), 3342–3347. 10.1128/AAC.02437-14 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

