Safety and Efficacy of Adjunctive Therapy With Artesunate in the Treatment of Severe Malaria: A Systematic Review and Meta-Analysis
- PMID: 33343367
- PMCID: PMC7748123
- DOI: 10.3389/fphar.2020.596697
Safety and Efficacy of Adjunctive Therapy With Artesunate in the Treatment of Severe Malaria: A Systematic Review and Meta-Analysis
Abstract
Objective: The purpose of this meta-analysis of longitudinal studies is to determine the safety and efficacy of artesunate combined with other forms of adjunctive therapies for severe malaria. Methods: Following the PRISMA guidelines, we searched multiple databases with the search terms "artesunate" and "adjunctive therapy" and "severe malaria" in July 2020. If the search showed a randomized controlled trial, the study was included in this meta-analysis. The random-effects model was used to calculate the combined incidence rate and relative risk or risk difference. Results: This meta-analysis included nine longitudinal studies with 724 participants. We found that the mortality rates in the artesunate monotherapy group and the artesunate + adjuvant therapy group are similar (RD = -0.02, 95% confidence interval: -0.06-0.02). The incidence of adverse reactions in the artesunate monotherapy group and the artesunate + adjuvant therapy group was also similar. Conclusion: No significant differences in safety and efficacy were observed between the artesunate monotherapy group and the artesunate + adjuvant therapy group. Higher quality and rigorously designed randomized controlled studies are needed to validate our findings.
Keywords: artesunate; efficacy; meta-analysis; safety; severe malaria; systematic review.
Copyright © 2020 Zou, Tuo, Zhang, Guo, Yuan, Zhang, Xu, Pan, Tang, Deng, Juli, Wu, Guo, Li, Huang, Xu, Song and Wang.
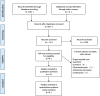
Figures





References
-
- Anstey N. M., Weinberg J. B., Hassanali M. Y., Mwaikambo E. D., Manyenga D., Misukonis M. A., et al. (1996). Nitric oxide in tanzanian children with malaria: inverse relationship between malaria severity and nitric oxide production/nitric oxide synthase type 2 expression. J. Exp. Med. 184 (2), 557–567. 10.1084/jem.184.2.557. - DOI - PMC - PubMed
-
- Charunwatthana P., Abul Faiz M., Ruangveerayut R., Maude R. J., Rahman M. R., Roberts L. J., et al. (2009). N-acetylcysteine as adjunctive treatment in severe malaria: a randomized, double-blinded placebo-controlled clinical trial. Crit. Care Med. 37 (2), 516–522. 10.1097/CCM.0b013e3181958dfd. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

