Ficus carica L. Attenuates Denervated Skeletal Muscle Atrophy via PPARα/NF-κB Pathway
- PMID: 33343385
- PMCID: PMC7744683
- DOI: 10.3389/fphys.2020.580223
Ficus carica L. Attenuates Denervated Skeletal Muscle Atrophy via PPARα/NF-κB Pathway
Abstract
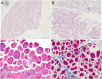
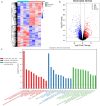
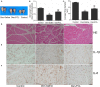
Treatment options for denervated skeletal muscle atrophy are limited, in part because the underlying molecular mechanisms are not well understood. Unlike previous transcriptomics studies conducted in rodent models of peripheral nerve injury, in the present study, we performed high-throughput sequencing with denervated atrophic biceps muscle and normal (non-denervated) sternocleidomastoid muscle samples obtained from four brachial plexus injury (BPI) patients. We also investigated whether Ficus carica L. (FCL.) extract can suppress denervated muscle atrophy in a mouse model, along with the mechanism of action. We identified 1471 genes that were differentially expressed between clinical specimens of atrophic and normal muscle, including 771 that were downregulated and 700 that were upregulated. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses revealed that the differentially expressed genes were mainly enriched in the GO terms "structural constituent of muscle," "Z disc," "M band," and "striated muscle contraction," as well as "Cell adhesion molecules," "Glycolysis/Gluconeogenesis," "Peroxisome proliferator-activated receptor alpha (PPARα) signaling pathway," and "P53 signaling pathway." In experiments using mice, the reduction in wet weight and myofiber diameter in denervated muscle was improved by FCL. extract compared to saline administration, which was accompanied by downregulation of the proinflammatory cytokines interleukin (IL)-1β and IL-6. Moreover, although both denervated groups showed increased nuclear factor (NF)-κB activation and PPARα expression, the degree of NF-κB activation was lower while PPARα and inhibitor of NF-κB IκBα expression was higher in FCL. extract-treated mice. Thus, FCL. extract suppresses denervation-induced inflammation and attenuates muscle atrophy by enhancing PPARα expression and inhibiting NF-κB activation. These findings suggest that FCL. extract has therapeutic potential for preventing denervation-induced muscle atrophy caused by peripheral nerve injury or disease.
Keywords: Ficus carica; NF-κß; PPAR; denervated muscle atrophy; muscle atrophy; peripheral nerve injury.
Copyright © 2020 Dai, Xiang, Fu, Xu, Jiang and Xu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

