From Entry to Egress: Strategic Exploitation of the Cellular Processes by HIV-1
- PMID: 33343516
- PMCID: PMC7746852
- DOI: 10.3389/fmicb.2020.559792
From Entry to Egress: Strategic Exploitation of the Cellular Processes by HIV-1
Abstract
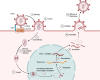
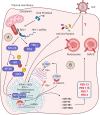
HIV-1 employs a rich arsenal of viral factors throughout its life cycle and co-opts intracellular trafficking pathways. This exquisitely coordinated process requires precise manipulation of the host microenvironment, most often within defined subcellular compartments. The virus capitalizes on the host by modulating cell-surface proteins and cleverly exploiting nuclear import pathways for post entry events, among other key processes. Successful virus-cell interactions are indeed crucial in determining the extent of infection. By evolving defenses against host restriction factors, while simultaneously exploiting host dependency factors, the life cycle of HIV-1 presents a fascinating montage of an ongoing host-virus arms race. Herein, we provide an overview of how HIV-1 exploits native functions of the host cell and discuss recent findings that fundamentally change our understanding of the post-entry replication events.
Keywords: HIV-1 infection; capsid uncoating; cell organelles; host-virus interactions; restriction factors.
Copyright © 2020 Ramdas, Sahu, Mishra, Bhardwaj and Chande.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Alsahafi N., Richard J., Prévost J., Coutu M., Brassard N., Parsons M. S., et al. (2017). Impaired downregulation of NKG2D ligands by Nef proteins from elite controllers sensitizes HIV-1-infected cells to antibody-dependent cellular cytotoxicity. J. Virol. 91:e00109-17 10.1128/JVI.00109-17 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

