Differential Skewing of Circulating MR1-Restricted and γδ T Cells in Human Psoriasis Vulgaris
- PMID: 33343564
- PMCID: PMC7744298
- DOI: 10.3389/fimmu.2020.572924
Differential Skewing of Circulating MR1-Restricted and γδ T Cells in Human Psoriasis Vulgaris
Abstract
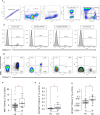
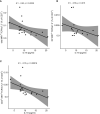
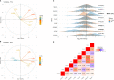
Psoriasis vulgaris (PV) is a chronic, recurrent inflammatory dermatosis mediated by aberrantly activated immune cells. The role of the innate-like T cells, particularly gammadelta T (γδT) cells and MR1-restricted T lymphocytes, is incompletely explored, mainly through animal models, or by use of surrogate lineage markers, respectively. Here, we used case-control settings, multiparameter flow cytometry, 5-OP-RU-loaded MR1-tetramers, Luminex technology and targeted qRT-PCR to dissect the cellular and transcriptional landscape of γδ and MR1-restricted blood T cells in untreated PV cases (n=21, 22 matched controls). High interpersonal differences in cell composition were observed, fueling transcriptional variability at healthy baseline. A minor subset of canonical CD4+CD8+MR1-tet+TCRVα7.2+ and CD4+CD8-MR1-tet+TCRVα7.2+ T cells was the most significantly underrepresented community in male PV individuals, whereas Vδ2+ γδ T cells expressing high levels of TCR and Vδ1-δ2- γδ T cells expressing intermediate levels of TCR were selectively enriched in affected males, partly reflecting disease severity. Our findings highlight a formerly unappreciated skewing of human circulating MAIT and γδ cytomes during PV, and reveal their compositional changes in relation to sex, CMV exposure, serum cytokine content, BMI, and inflammatory burden. Complementing numerical alterations, we finally show that flow-sorted, MAIT and γδ populations exhibit divergent transcriptional changes in mild type I psoriasis, consisting of differential bulk expression for signatures of cytotoxicity/type-1 immunity (EOMES, RUNX3, IL18R), type-3 immunity (RORC, CCR6), and T cell innateness (ZBTB16).
Keywords: MR1; cytokines; gammadelta T lymphocytes; mucosal associated invariant T cells; psoriasis.
Copyright © 2020 Plužarić, Štefanić, Mihalj, Tolušić Levak, Muršić, Glavaš-Obrovac, Petrek, Balogh and Tokić.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

