Neutrophils From Patients With Invasive Candidiasis Are Inhibited by Candida albicans Biofilms
- PMID: 33343568
- PMCID: PMC7747767
- DOI: 10.3389/fimmu.2020.587956
Neutrophils From Patients With Invasive Candidiasis Are Inhibited by Candida albicans Biofilms
Abstract
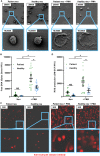
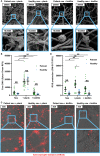
Invasive candidiasis frequently involves medical device placement. On the surfaces of these devices, Candida can form biofilms and proliferate in adherent layers of fungal cells surrounded by a protective extracellular matrix. Due in part to this extracellular matrix, biofilms resist host defenses and antifungal drugs. Previous work (using neutrophils from healthy donors) found that one mechanism employed to resist host defenses involves the inhibition of neutrophil extracellular traps (NET) formation. NETs contain nuclear DNA, as well as antimicrobial proteins that can ensnare pathogens too large or aggregated to be effectively killed by phagocytosis. Given that these neutrophil structures are anticipated to have activity against the large aggregates of C. albicans biofilms, understanding the role of this inhibition in patients could provide insight into new treatment strategies. However, prior work has not included patients. Here, we examine NET formation by neutrophils collected from patients with invasive candidiasis. When compared to neutrophils from healthy participants, we show that patient neutrophils exhibit a heightened background level of NET release and respond to a positive stimulus by producing 100% more NETs. However, despite these physiologic differences, patient neutrophil responses to C. albicans were similar to healthy neutrophils. For both groups, planktonic cells induce strong NET release and biofilms inhibit NET formation. These results show that a mechanism of immune evasion for fungal biofilms translates to the clinical setting.
Keywords: Candida; biofilm; invasive candidiasis; neutrophil; neutrophil extracellular trap; patients; reactive oxygen species.
Copyright © 2020 Kernien, Johnson, Bayless, Chovanec and Nett.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

