Angiogenesis in Lymph Nodes Is a Critical Regulator of Immune Response and Lymphoma Growth
- PMID: 33343570
- PMCID: PMC7744479
- DOI: 10.3389/fimmu.2020.591741
Angiogenesis in Lymph Nodes Is a Critical Regulator of Immune Response and Lymphoma Growth
Abstract
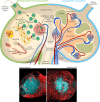
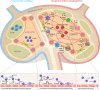
Tumor-induced remodeling of the microenvironment in lymph nodes (LNs) includes the formation of blood vessels, which goes beyond the regulation of metabolism, and shaping a survival niche for tumor cells. In contrast to solid tumors, which primarily rely on neo-angiogenesis, hematopoietic malignancies usually grow within pre-vascularized autochthonous niches in secondary lymphatic organs or the bone marrow. The mechanisms of vascular remodeling in expanding LNs during infection-induced responses have been studied in more detail; in contrast, insights into the conditions of lymphoma growth and lodging remain enigmatic. Based on previous murine studies and clinical trials in human, we conclude that there is not a universal LN-specific angiogenic program applicable. Instead, signaling pathways that are tightly connected to autochthonous and infiltrating cell types contribute variably to LN vascular expansion. Inflammation related angiogenesis within LNs relies on dendritic cell derived pro-inflammatory cytokines stimulating vascular endothelial growth factor-A (VEGF-A) expression in fibroblastic reticular cells, which in turn triggers vessel growth. In high-grade B cell lymphoma, angiogenesis correlates with poor prognosis. Lymphoma cells immigrate and grow in LNs and provide pro-angiogenic growth factors themselves. In contrast to infectious stimuli that impact on LN vasculature, they do not trigger the typical inflammatory and hypoxia-related stroma-remodeling cascade. Blood vessels in LNs are unique in selective recruitment of lymphocytes via high endothelial venules (HEVs). The dissemination routes of neoplastic lymphocytes are usually disease stage dependent. Early seeding via the blood stream requires the expression of the homeostatic chemokine receptor CCR7 and of L-selectin, both cooperate to facilitate transmigration of tumor and also of protective tumor-reactive lymphocytes via HEV structures. In this view, the HEV route is not only relevant for lymphoma cell homing, but also for a continuous immunosurveillance. We envision that HEV functional and structural alterations during lymphomagenesis are not only key to vascular remodeling, but also impact on tumor cell accessibility when targeted by T cell-mediated immunotherapies.
Keywords: B cell malignancy; angiogenesis; high endothelial venule; lymph node; lymphocyte trafficking; lymphoma; reactive endothelium; tumor microenvironment.
Copyright © 2020 Menzel, Höpken and Rehm.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

