Trends in the crossover of patients in phase III oncology clinical trials in the USA
- PMID: 33343701
- PMCID: PMC7738270
- DOI: 10.3332/ecancer.2020.1142
Trends in the crossover of patients in phase III oncology clinical trials in the USA
Abstract
Background: The incorporation of crossover in randomised controlled trials is accepted as an ethical obligation, especially in cancer clinical trials. The more common type of crossover is crossover allowance, which allows patients assigned to one arm to switch to another arm if there is an established benefit in the crossover arm. In contrast, crossover-designed studies involve switching patients from all arms to a different arm as part of the study design. Crossover allowance may have advantages in patient recruitment and incorporating crossover after initial positive results fulfil ethical requirements. However, crossover can also contribute to confounding major endpoints of studies, such as overall survival or the second progression-free survival interval. For this reason, it is important to investigate and identify potential trends of crossover in clinical trials testing novel therapies.
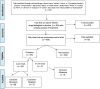
Methods: Data about cancer clinical trials were extracted from clinicaltrials.gov. The search query was limited to completed phase III studies in adult populations. Location was limited to the USA. Date range extended from 1990 to 2019. Search query included the terms: cancer; completed- recruitment status; age: 18-65+ years; sex: all; location: USA; and study phase: phase 3. Studies were then excluded if they were not randomised controlled trials (RCTs) with the primary purpose of treatment and if they did not test cancer-related interventions.
Results: A total of 744 clinical trials were identified. There were 459 RCTs aimed at treatment, and of those, 35 utilised crossover. The start dates of these crossover trials ranged from 1997 to 2012. Thirty studies utilised crossover allowance. Prostate, breast and gastrointestinal stromal tumour cancers were the most represented cancer types in crossover studies. Among the 30 studies, the median proportion of patients who crossed over relative to the original arm assignment ranged from 2% to 88%, with a median of 57.5%.
Conclusions: The proportion of identified clinical trials with crossover compared to those without is extremely small. Crossover in clinical trials studying cancer treatment does not appear to be a widespread practice. Even though statistical approaches to mitigate confounding exist, crossover can still skew accurate reporting of the impact of experimental therapies on overall survival.
Keywords: cancer; controlled trials; crossover; randomised.
© the authors; licensee ecancermedicalscience.
Conflict of interest statement
None.
Figures
References
LinkOut - more resources
Full Text Sources