Re-appraising the potential of naringin for natural, novel orthopedic biotherapies
- PMID: 33343723
- PMCID: PMC7727086
- DOI: 10.1177/1759720X20966135
Re-appraising the potential of naringin for natural, novel orthopedic biotherapies
Abstract
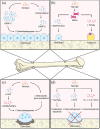
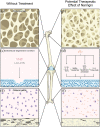
Naringin is a naturally occurring flavonoid found in plants of the Citrus genus that has historically been used in traditional Chinese medical regimens for the treatment of osteoporosis. Naringin modulates signaling through numerous molecular pathways critical to musculoskeletal development, cellular differentiation, and inflammation. Administration of naringin increases in vitro expression of bone morphogenetic proteins (BMPs) and activation of the Wnt/β-catenin and extracellular signal-related kinase (Erk) pathways, thereby promoting osteoblastic proliferation and differentiation from stem cell precursors for bone formation. Naringin also inhibits osteoclastogenesis by both modifying RANK/RANKL interactions and inducing apoptosis in osteoclasts in vitro. In addition, naringin acts on the estrogen receptor in bone to mimic the native bone-preserving effects of estrogen, with few systemic side effects on other estrogen-sensitive tissues. The efficacy of naringin therapy in reducing the osteolysis characteristic of common musculoskeletal pathologies such as osteoporosis, degenerative joint disease, and osteomyelitis, as well as inflammatory conditions affecting bone such as diabetes mellitus, has been extensively demonstrated in vitro and in animal models. Naringin thus represents a naturally abundant, cost-efficient agent whose potential for use in novel musculoskeletal biotherapies warrants re-visiting and further exploration through human studies. Here, we review the cellular mechanisms of action that have been elucidated regarding the action of naringin on bone resident cells and the bone microenvironment, in vivo evidence of naringin's osteostimulative and chondroprotective properties in the setting of osteolytic bone disease, and current limitations in the development of naringin-containing translational therapies for common musculoskeletal conditions.
Keywords: diabetes mellitus; naringin; osteoarthritis; osteoclastogenesis; osteomyelitis; osteoporosis.
© The Author(s), 2020.
Conflict of interest statement
Conflict of interest statement: The authors declare that there is no conflict of interest.
Figures
References
-
- Taleb-Contini SH, Salvador MJ, Balanco JM, et al. Antiprotozoal effect of crude extracts and flavonoids isolated from Chromolaena hirsuta (asteraceae). Phytother Res 2004; 18: 250–254. - PubMed
-
- Gonzalez R, Ballester I, Lopez-Posadas R, et al. Effects of flavonoids and other polyphenols on inflammation. Crit Rev Food Sci Nutr 2011; 51: 331–362. - PubMed
-
- Bharti S, Rani N, Krishnamurthy B, et al. Preclinical evidence for the pharmacological actions of naringin: a review. Planta Med 2014; 80: 437–451. - PubMed
-
- Wei M, Yang Z, Li P, et al. Anti-osteoporosis activity of naringin in the retinoic acid-induced osteoporosis model. Am J Chin Med 2007; 35: 663–667. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

