Skin manifestations in spondyloarthritis
- PMID: 33343725
- PMCID: PMC7727049
- DOI: 10.1177/1759720X20975915
Skin manifestations in spondyloarthritis
Abstract
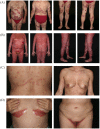
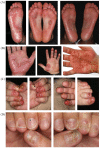
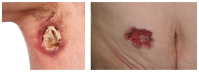
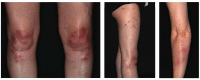
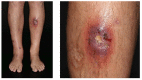
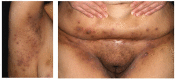
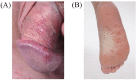
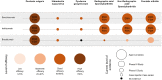
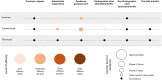
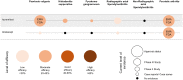
Spondyloarthritides (SpA) like psoriatic arthritis, axial spondyloarthritis/ankylosing spondylitis, reactive arthritis and inflammatory bowel disease (IBD)-associated SpA can present with characteristic skin manifestations. These SpA-associated skin disorders may precede joint involvement, reflect a loss of efficacy of a current systemic treatment or can even be treatment associated. Cutaneous manifestations in SpA not only add additional morbidity with physical impact but also impose a psychosocial burden on affected patients. Psoriasis (PsO) - the main skin disease in SpA - has a variety of clinical presentations, including plaque-type PsO, inverse PsO, guttate PsO, erythrodermic PsO, nail PsO and pustular types. SpA associated with IBD presents with neutrophilic and granulomatous skin disorders, including pyoderma gangrenosum, hidradenitis suppurativa and cutaneous Crohn's disease. Reactive arthritides has a favourable prognosis and may feature keratoderma blenorrhagicum or balanitis circinatum as typical skin manifestations. Immunologically, SpA-associated skin diseases share interleukin (IL)-17 and IL-23 dysregulation but show distinctive genetic and immunological profiles. Therefore, they vary in their treatment responses to targeted therapies with biologicals or small molecules. In this review, we highlight the clinical presentation of skin manifestations in SpA and discuss therapeutic approaches in this interdisciplinary field.
Keywords: erythema nodosum; hidradenitis suppurativa; psoriasis; psoriatic arthritis; pyoderma gangrenosum; skin; spondyloarthritis.
© The Author(s), 2020.
Conflict of interest statement
Conflict of interest statement: KM has received honoraria or travel expenses for lecture and research activities from Abbvie, Biogen, Celgene, Janssen-Cilag and UCB Pharma. AS has received travel expenses from Janssen-Cilag and Celgene DP has received grant/research support from AbbVie, Lilly, MSD, Novartis, and Pfizer; has been a consultant for AbbVie, Biocad, Gilead, GSK, Lilly, MSD, Novartis, Pfizer, Samsung and UCB; and served on the speakers bureau for AbbVie, BMS, Lilly, MSD, Novartis, Pfizer and UCB. KG has received honoraria or travel expenses for lecture and research activities from Abbvie, Almirall, Biogen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Delenex, Eli Lilly, Galderma, Janssen-Cilag, Medac, MSD, Novartis, Pfizer and UCB Pharma.
Figures




Similar articles
-
Skin Manifestations of Rheumatoid Arthritis, Juvenile Idiopathic Arthritis, and Spondyloarthritides.Clin Rev Allergy Immunol. 2017 Dec;53(3):371-393. doi: 10.1007/s12016-017-8632-5. Clin Rev Allergy Immunol. 2017. PMID: 28752373 Review.
-
[The role of biologic therapy in the treatment of extraintestinal manifestations and complications of inflammatory bowel disease].Acta Med Croatica. 2013 Apr;67(2):195-201. Acta Med Croatica. 2013. PMID: 24471303 Review. Croatian.
-
Acute anterior uveitis and other extra-articular manifestations of spondyloarthritis.J Med Life. 2015 Jul-Sep;8(3):319-25. J Med Life. 2015. PMID: 26351533 Free PMC article.
-
Etanercept in Axial Spondyloarthritis, Psoriatic Arthritis, and Plaque Psoriasis: Real-World Outcome Data from German Non-interventional Study ADEQUATE.Rheumatol Ther. 2024 Apr;11(2):331-348. doi: 10.1007/s40744-023-00633-2. Epub 2024 Feb 3. Rheumatol Ther. 2024. PMID: 38308727 Free PMC article.
-
Treatment Patterns and Healthcare Resource Utilization Among Newly Diagnosed Psoriasis, Psoriatic Arthritis, Axial Spondyloarthritis, and Hidradenitis Suppurativa Patients with Past Diagnosis of an Inflammatory Condition: A Retrospective Cohort Analysis of Claims Data in the United States.Adv Ther. 2023 Oct;40(10):4358-4376. doi: 10.1007/s12325-023-02558-2. Epub 2023 Jul 24. Adv Ther. 2023. PMID: 37486558 Free PMC article.
Cited by
-
[Spondyloarthritis in childhood and adulthood].Z Rheumatol. 2022 Feb;81(1):14-21. doi: 10.1007/s00393-021-01135-8. Epub 2022 Jan 5. Z Rheumatol. 2022. PMID: 34985566 Review. German.
-
Divergent B-cell and cytotoxic TNK cell activation signatures in HLA-B27-associated ankylosing spondylitis and acute anterior uveitis.Front Immunol. 2025 Mar 7;16:1546429. doi: 10.3389/fimmu.2025.1546429. eCollection 2025. Front Immunol. 2025. PMID: 40124359 Free PMC article.
-
[Granulomatous dermatoses].Z Rheumatol. 2022 Sep;81(7):577-586. doi: 10.1007/s00393-022-01239-9. Epub 2022 Jul 19. Z Rheumatol. 2022. PMID: 35854155 Review. German.
-
Potential effects of shift work on skin autoimmune diseases.Front Immunol. 2023 Feb 14;13:1000951. doi: 10.3389/fimmu.2022.1000951. eCollection 2022. Front Immunol. 2023. PMID: 36865523 Free PMC article. Review.
-
The role of the multidisciplinary team in the management of psoriatic arthritis.Musculoskeletal Care. 2022 Nov;20 Suppl 1(Suppl 1):S32-S40. doi: 10.1002/msc.1690. Musculoskeletal Care. 2022. PMID: 36356109 Free PMC article. Review.
References
-
- Braun J, Bollow M, Remlinger G, et al. Prevalence of spondylarthropathies in HLA-B27 positive and negative blood donors. Arthritis Rheum 1998; 41: 58–67. - PubMed
-
- Rudwaleit M, van der Heijde D, Landewé R, et al. The development of assessment of spondyloarthritis international society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 2009; 68: 777–783. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

