Socio-economic burden and resource utilisation in Italian patients with chronic urticaria: 2-year data from the AWARE study
- PMID: 33343800
- PMCID: PMC7726718
- DOI: 10.1016/j.waojou.2020.100470
Socio-economic burden and resource utilisation in Italian patients with chronic urticaria: 2-year data from the AWARE study
Abstract
Introduction: In Italy, the real-world evidence on the extent of adherence to guidelines and the benefits of recommended therapeutic medications and their impact on the quality of life (QoL) of H1-antihistamines (H1-AH) refractory chronic urticaria (CU) patients is limited.
Methods: AWARE (A World-wide Antihistamine-Refractory chronic urticaria patient Evaluation) was a global prospective, non-interventional study of CU in real-world setting which included patients aged ≥18 years with a medically confirmed diagnosed of CU present for more than 2 months. In this study, the disease characteristics, pharmacological treatments and patient-reported outcomes (PROs) are reported.
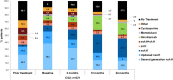
Results: In total, 159 patients from 24 study centres in Italy completed the study. At baseline, 221 (89.5%) and 8 (3.2%) patients had chronic spontaneous urticaria (CSU) and chronic inducible urticaria (CIndU), respectively, while 18 (7.3%) patients had concomitant CSU and CIndU. For CSU patients, mean dermatology life quality index and CU quality of life questionnaire scores reduced to 3.0 ± 4.9 and 14.6 ± 18.6 at Month 24 from baseline scores of 7.5 ± 6.6 and 33.2 ± 19.5, respectively, indicating an improvement in QoL. This was reflected in their work-life as work productivity impairment reduced considerably after 2 years. Only 71.9% CSU patients had a prior treatment, while during the study, 96.8% of the patients were treated with a medication. At baseline, only 52.9% CSU patients reported nonsedating H1-antihistamines as first-line of treatment in prior medication, this increased to 89.6% during current medication.
Conclusion: This study shows that CSU has a considerable socio-economic burden and an improvement in QoL can be achieved in CSU patients if an appropriate therapeutic path is followed.
Keywords: AWARE study; CIndU, chronic inducible urticaria; CSU, chronic spontaneous urticaria; CU, chronic urticaria; CU-Q2oL, CU quality of life questionnaire; Chronic spontaneous urticaria; DLQI, dermatology life quality index; GCP, good clinical practices; H1-AH, H1-antihistamines; Italy; PRO, patient-reported outcomes; QoL, quality of life; Resource utilisation; SD, standard deviation; Socio-economic burden; UAS7, weekly urticaria activity score; WPAI-CU, work productivity and activity impairment questionnaire; nsAH, non-sedating H1-AH; sAH, sedating H1-AH.
© 2020 The Author(s).
Conflict of interest statement
O Rossi: Alk-abello, Italchimici and GSK. E Iemoli: Served as a medical doctor in ASST FBF-Sacco Milano. Prof A Patrizi: Principal investigator for Abbvie, Eli Lilly, Leo, Novartis, Sanofi Genzyme Regeneron, Pfizer and speaker, advisory board member and/or consultant for Sanofi Genzyme Regeneron, Menarini, Abblie, Lilly, Pierre fabre, La roche Posay, Novartis, Leo, Almirall, Celgene. L Stingeni: Principal investigator in clinical trials sponsored by Novartis and has served on advisory board from Novartis. S Calvieri: Abiogen. M Gola: Sanofi e Beiersdorf. P Dapavo: Novartis, Abbvie, Celgene, Lilly spa, Leopharma, Sandoz, Janssen. L Zichichi: Principal Investigator in clinical trials sponsored by Novartis and has served on advisory board from Novartis. F Saccheri: Employee, Novartis. All other authors have noting to disclose.
Figures




Similar articles
-
Clinical characteristics and management of chronic spontaneous urticaria in patients refractory to H1-Antihistamines in Asia, Middle-East and Africa: Results from the AWARE-AMAC study.World Allergy Organ J. 2020 Apr 30;13(4):100117. doi: 10.1016/j.waojou.2020.100117. eCollection 2020 Apr. World Allergy Organ J. 2020. PMID: 32382379 Free PMC article.
-
Chronic urticaria treatment patterns and changes in quality of life: AWARE study 2-year results.World Allergy Organ J. 2020 Sep 12;13(9):100460. doi: 10.1016/j.waojou.2020.100460. eCollection 2020 Sep. World Allergy Organ J. 2020. PMID: 32983330 Free PMC article.
-
H1-antihistamine-refractory chronic spontaneous urticaria: it's worse than we thought - first results of the multicenter real-life AWARE study.Clin Exp Allergy. 2017 May;47(5):684-692. doi: 10.1111/cea.12900. Epub 2017 Mar 2. Clin Exp Allergy. 2017. PMID: 28160338 Clinical Trial.
-
The global burden of chronic urticaria for the patient and society.Br J Dermatol. 2021 Feb;184(2):226-236. doi: 10.1111/bjd.19561. Epub 2020 Nov 2. Br J Dermatol. 2021. PMID: 32956489 Review.
-
Expert consensus on the use of omalizumab in chronic urticaria in China.World Allergy Organ J. 2021 Dec 5;14(11):100610. doi: 10.1016/j.waojou.2021.100610. eCollection 2021 Nov. World Allergy Organ J. 2021. PMID: 34934470 Free PMC article. Review.
Cited by
-
Burden of chronic spontaneous urticaria in Italy through healthcare resource utilization and direct costs: a retrospective analysis of real-world using administrative healthcare data.BMC Health Serv Res. 2025 Jul 23;25(1):969. doi: 10.1186/s12913-025-13122-9. BMC Health Serv Res. 2025. PMID: 40696317 Free PMC article.
-
Urticaria Voices: Real-World Treatment Patterns and Outcomes in Chronic Spontaneous Urticaria.Dermatol Ther (Heidelb). 2025 Aug;15(8):2201-2215. doi: 10.1007/s13555-025-01461-8. Epub 2025 Jun 22. Dermatol Ther (Heidelb). 2025. PMID: 40544394 Free PMC article.
-
Chronic inducible urticaria - having more than one is common and clinically relevant.Front Immunol. 2025 Jun 30;16:1584771. doi: 10.3389/fimmu.2025.1584771. eCollection 2025. Front Immunol. 2025. PMID: 40661948 Free PMC article.
-
Clinical profile, prevalence, and burden of chronic spontaneous urticaria in the United States.World Allergy Organ J. 2025 Jul 18;18(8):101081. doi: 10.1016/j.waojou.2025.101081. eCollection 2025 Aug. World Allergy Organ J. 2025. PMID: 40718158 Free PMC article.
References
-
- Zuberbier T., Aberer W., Asero R. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393–1414. - PubMed
-
- Maurer M., Weller K., Bindslev-Jensen C. Unmet clinical needs in chronic spontaneous urticaria. A GA(2)LEN task force report. Allergy. 2011;66:317–330. - PubMed
-
- Greaves M.W. Chronic idiopathic urticaria. Curr Opin Allergy Clin Immunol. 2003;3:363–368. - PubMed
-
- Buss Y.A., Garrelfs U.C., Sticherling M. Chronic urticaria--which clinical parameters are pathogenetically relevant? A retrospective investigation of 339 patients. J Dtsch Dermatol Ges. 2007;5:22–29. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

