Melatonin Suppresses Ferroptosis Induced by High Glucose via Activation of the Nrf2/HO-1 Signaling Pathway in Type 2 Diabetic Osteoporosis
- PMID: 33343809
- PMCID: PMC7732386
- DOI: 10.1155/2020/9067610
Melatonin Suppresses Ferroptosis Induced by High Glucose via Activation of the Nrf2/HO-1 Signaling Pathway in Type 2 Diabetic Osteoporosis
Abstract
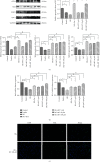
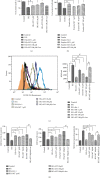
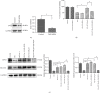
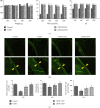
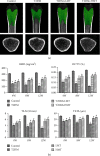
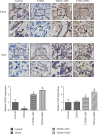
Ferroptosis is recently identified, an iron- and reactive oxygen species- (ROS-) dependent form of regulated cell death. This study was designed to determine the existence of ferroptosis in the pathogenesis of type 2 diabetic osteoporosis and confirm that melatonin can inhibit the ferroptosis of osteoblasts through activating Nrf2/HO-1 signaling pathway to improve bone microstructure in vivo and in vitro. We treated MC3T3-E1 cells with different concentrations of melatonin (1, 10, or 100 μM) and exposed them to high glucose (25.5 mM) for 48 h in vitro. Our data showed that high glucose can induce osteoblast cytotoxicity and the accumulation of lipid peroxide, the mitochondria of osteoblast show the same morphology changes as the erastin treatment group, and the expression of ferroptosis-related proteins glutathione peroxidase 4 (GPX4) and cystine-glutamate antiporter (SLC7A11) is downregulated, but these effects were reversed by ferroptosis inhibitor ferrastatin-1 and iron chelator deferoxamine (DFO). Furthermore, western blot and real-time polymerase chain reaction were used to detect the expression levels of nuclear factor erythroid 2-related factor 2 (Nrf2) and heme oxygenase-1 (HO-1); osteogenic capacity was evaluated by alizarin red S staining and the expression of osteoprotegerin, osteocalcin, and alkaline phosphatase; the results showed that the expression levels of these proteins in osteoblasts with 1, 10, or 100 μM melatonins were significantly higher than the high glucose group, but after using Nrf2-SiRNA interference, the therapeutic effect of melatonin was significantly inhibited. We also performed in vivo experiments in a diabetic rat model treated with two concentrations of melatonin (10, 50 mg/kg). Dynamic bone histomorphometry and micro-CT were used to observe the rat bone microstructure, and the expression of GPX4 and Nrf2 was determined by immunohistochemistry. Here, we first report that high glucose induces ferroptosis via increased ROS/lipid peroxidation/glutathione depletion in type 2 diabetic osteoporosis. More importantly, melatonin significantly reduced the level of ferroptosis and improved the osteogenic capacity of MC3T3-E1 through activating the Nrf2/HO-1 pathway in vivo and in vitro.
Copyright © 2020 Hongdong Ma et al.
Conflict of interest statement
The authors declare no competing financial interests. No benefits in any form have been or will be received from a commercial party directly or indirectly by the authors of this article.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

