The Actin Cytoskeleton as an Active Adaptive Material
- PMID: 33343823
- PMCID: PMC7748259
- DOI: 10.1146/annurev-conmatphys-031218-013231
The Actin Cytoskeleton as an Active Adaptive Material
Abstract
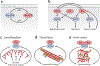
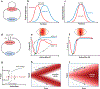
Actin is the main protein used by biological cells to adapt their structure and mechanics to their needs. Cellular adaptation is made possible by molecular processes that strongly depend on mechanics. The actin cytoskeleton is also an active material that continuously consumes energy. This allows for dynamical processes that are possible only out of equilibrium and opens up the possibility for multiple layers of control that have evolved around this single protein.Here we discuss the actin cytoskeleton from the viewpoint of physics as an active adaptive material that can build structures superior to man-made soft matter systems. Not only can actin be used to build different network architectures on demand and in an adaptive manner, but it also exhibits the dynamical properties of feedback systems, like excitability, bistability, or oscillations. Therefore, it is a prime example of how biology couples physical structure and information flow and a role model for biology-inspired metamaterials.
Keywords: active matter; cell mechanics; feedback control; metamaterial; nonequilibrium physics; soft matter.
Figures
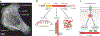



References
-
- Alberts B, Johnson A, Lewis J, Morgan D, Raff M, et al. 2014. Molecular Biology of the Cell. New York: Norton & Co; 6th rev. ed.
-
- Phillips R, Kondev J, Theriot J, Garcia H. 2012. Physical Biology of the Cell. New York: Taylor & Francis; 2nd ed.
-
- Jockusch BM, ed. 2017. The Actin Cytoskeleton. Berlin: Springer
Grants and funding
LinkOut - more resources
Full Text Sources
