C2N: A Class of Covalent Frameworks with Unique Properties
- PMID: 33344122
- PMCID: PMC7740084
- DOI: 10.1002/advs.202001767
C2N: A Class of Covalent Frameworks with Unique Properties
Abstract
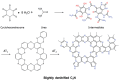
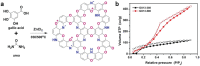
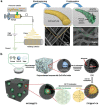
C2N is a unique member of the CnNm family (carbon nitrides), i.e., having a covalent structure that is ideally composed of carbon and nitrogen with only 33 mol% of nitrogen. C2N, with a stable composition, can easily be prepared using a number of precursors. Moreover, it is currently gaining extensive interest owing to its high polarity and good thermal and chemical stability, complementing carbon as well as classical carbon nitride (C3N4) in various applications, such as catalysis, environmental science, energy storage, and biotechnology. In this review, a comprehensive overview on C2N is provided; starting with its preparation methods, followed by a fundamental understanding of structure-property relationships, and finally introducing its application in gas sorption and separation technologies, as supercapacitor and battery electrodes, and in catalytic and biological processes. The review with an outlook on current research questions and future possibilities and extensions based on these material concepts is ended.
Keywords: C2N; applications; carbon materials; heteroatoms; regular pores.
© 2020 The Authors. Published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Sakaushi K., Antonietti M., Acc. Chem. Res. 2015, 48, 1591. - PubMed
-
- Fechler N., Zussblatt N. P., Rothe R., Schlogl R., Willinger M. G., Chmelka B. F., Antonietti M., Adv. Mater. 2016, 28, 1287. - PubMed
-
- King T. C., Matthews P. D., Holgado J. P., Jefferson D. A., Lambert R. M., Alavi A., Wright D. S., Carbon 2013, 64, 6.
-
- Yang S., Li W., Ye C., Wang G., Tian H., Zhu C., He P., Ding G., Xie X., Liu Y., Lifshitz Y., Lee S. T., Kang Z., Jiang M., Adv. Mater. 2017, 29, 1605625. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Medical
