Transient Receptor Potential Melastatin 8 (TRPM8) Channel Regulates Proliferation and Migration of Breast Cancer Cells by Activating the AMPK-ULK1 Pathway to Enhance Basal Autophagy
- PMID: 33344232
- PMCID: PMC7746826
- DOI: 10.3389/fonc.2020.573127
Transient Receptor Potential Melastatin 8 (TRPM8) Channel Regulates Proliferation and Migration of Breast Cancer Cells by Activating the AMPK-ULK1 Pathway to Enhance Basal Autophagy
Abstract
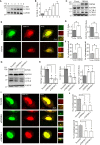
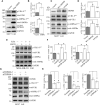
The calcium-permeable cation channel TRPM8 (transient receptor potential melastatin 8) is a member of the TRP superfamily of cation channels that is upregulated in various types of cancer with high levels of autophagy, including prostate, pancreatic, breast, lung, and colon cancers. Autophagy is closely regulated by AMP-activated protein kinase (AMPK) and plays an important role in tumor growth by generating nutrients through degradation of intracellular structures. Additionally, AMPK activity is regulated by intracellular Ca2+ concentration. Considering that TRPM8 is a non-selective Ca2+-permeable cation channel and plays a key role in calcium homoeostasis, we hypothesized that TRPM8 may control AMPK activity thus modulating cellular autophagy to regulate the proliferation and migration of breast cancer cells. In this study, overexpression of TRPM8 enhanced the level of basal autophagy, whereas TRPM8 knockdown reduced the level of basal autophagy in several types of mammalian cancer cells. Moreover, the activity of the TRPM8 channel modulated the level of basal autophagy. The mechanism of regulation of autophagy by TRPM8 involves autophagy-associated signaling pathways for activation of AMPK and ULK1 and phagophore formation. Impaired AMPK abolished TRPM8-dependent regulation of autophagy. TRPM8 interacts with AMPK in a protein complex, and cytoplasmic C-terminus of TRPM8 mediates the TRPM8-AMPK interaction. Finally, basal autophagy mediates the regulatory effects of TRPM8 on the proliferation and migration of breast cancer cells. Thus, this study identifies TRPM8 as a novel regulator of basal autophagy in cancer cells acting by interacting with AMPK, which in turn activates AMPK to activate ULK1 in a coordinated cascade of TRPM8-mediated breast cancer progression.
Keywords: AMP-activated protein kinase(AMPK); LC3B; autophagy; cancer; transient receptor potential melastatin 8 (TRPM8).
Copyright © 2020 Huang, Li, Jia, Zhao, Zhou, Zhang, Ali, Michalak, Chen and Tang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

