Magnolol Suppresses Pancreatic Cancer Development In Vivo and In Vitro via Negatively Regulating TGF-β/Smad Signaling
- PMID: 33344246
- PMCID: PMC7738609
- DOI: 10.3389/fonc.2020.597672
Magnolol Suppresses Pancreatic Cancer Development In Vivo and In Vitro via Negatively Regulating TGF-β/Smad Signaling
Abstract
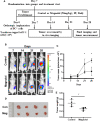
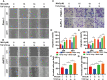
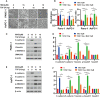
Magnolol, a hydroxylated biphenyl extracted from Magnolia officinalis, has recently drawn attention due to its anticancer potential. The present study was aimed to explore the effects of Magnolol on restraining the proliferation, migration and invasion of pancreatic cancer in vivo and in vitro. Magnolol showed significant anti-growth effect in an orthotopic xenograft nude mouse model, and immunohistochemical staining of the xenografts revealed that Magnolol suppressed vimentin expression and facilitated E-cadherin expression. The cytoactive detection using CCK-8 assay showed Magnolol inhibited PANC-1 and AsPC-1 concentration-dependently. Scratch healing assay and the Transwell invasion assay proved the inhibiting effects of Magnolol on cellular migration and invasion at a non-cytotoxic concentration. Western blot and rt-PCR showed that Magnolol suppressed epithelial-mesenchymal-transition by increasing the expression level of E-cadherin and decreasing those of N-cadherin and vimentin. Magnolol suppressed the TGF-β/Smad pathway by negatively regulating phosphorylation of Smad2/3. Moreover, TGF-β1 impaired the antitumor effects of Magnolol in vivo. These results demonstrated that Magnolol can inhibit proliferation, migration and invasion in vivo and in vitro by suppressing the TGF-β signal pathway and EMT. Magnolol could be a hopeful therapeutic drug for pancreatic malignancy.
Keywords: Smad; TGF-β; epithelial-mesenchymal-transition; magnolol; pancreatic cancer.
Copyright © 2020 Chen, Shen, Zhao, Wang, Shan, Li, Xu, Chen, Liu and Cao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
LinkOut - more resources
Full Text Sources
Research Materials

