Behind the Scenes: Nod-Like Receptor X1 Controls Inflammation and Metabolism
- PMID: 33344269
- PMCID: PMC7746548
- DOI: 10.3389/fcimb.2020.609812
Behind the Scenes: Nod-Like Receptor X1 Controls Inflammation and Metabolism
Abstract
Regulatory Nod-like receptors (NLRs) are a subgroup of the cytosolic NLR family of pathogen recognition receptors (PRRs). These receptors can tune the innate immune responses triggered by the activation of other PRRs by either augmenting or attenuating the activated pro-inflammatory signaling cascades. Nod-like receptor X1 (NLRX1) is the only known mitochondria-associated negative regulatory NLR. NLRX1 attenuates several inflammatory pathways and modulates cellular processes such as autophagy and mitochondrial function following infection or injury. Using both in vitro expression and in vivo experimental models, NLRX1 is extensively described in the context of anti-viral signaling and host-defense against invading pathogens. More recently, NLRX1 has also gained interest in the field of cancer and metabolism where NLRX1 functions to attenuate overzealous inflammation in various inflammatory and autoimmune diseases. However, the exact function of this novel receptor is still under debate and many, often contradictory, mechanisms of action together with cellular localizations have been proposed. Thus, a better understanding of the underlying mechanism is crucial for future research and development of novel therapeutical approaches. Here, we summarize the current findings on NLRX1 and discuss its role in both infectious and inflammatory context.
Keywords: infection; inflammation; metabolism; mitochondria; nod-like receptor X1.
Copyright © 2020 Snäkä and Fasel.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
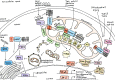
Figures

References
-
- Abdul-Sater A. A., Said-Sadier N., Lam V. M., Singh B., Pettengill M. A., Soares F., et al. (2010). Enhancement of reactive oxygen species production and chlamydial infection by the mitochondrial Nod-like family member NLRX1. J. Biol. Chem. 285, 41637–41645. 10.1074/jbc.M110.137885 - DOI - PMC - PubMed
-
- Aikawa C., Nakajima S., Karimine M., Nozawa T., Minowa-Nozawa A., Toh H., et al. (2018). NLRX1 Negatively Regulates Group A Streptococcus Invasion and Autophagy Induction by Interacting With the Beclin 1-UVRAG Complex. Front. Cell Infect. Microbiol. 8, 403. 10.3389/fcimb.2018.00403 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

