7,3',4'-Trihydroxyisoflavone, a Metabolite of the Soy Isoflavone Daidzein, Suppresses α-Melanocyte-Stimulating Hormone-Induced Melanogenesis by Targeting Melanocortin 1 Receptor
- PMID: 33344501
- PMCID: PMC7747307
- DOI: 10.3389/fmolb.2020.577284
7,3',4'-Trihydroxyisoflavone, a Metabolite of the Soy Isoflavone Daidzein, Suppresses α-Melanocyte-Stimulating Hormone-Induced Melanogenesis by Targeting Melanocortin 1 Receptor
Abstract
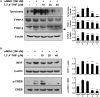
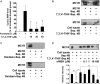
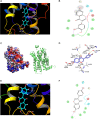
7,3',4'-Trihydroxyisoflavone (7,3',4'-THIF) is a metabolite of daidzein which is a representative isoflavone found in soybean. Recent studies suggested that 7,3',4'-THIF exerts a hypopigmentary effect in B16F10 cells, however, its underlying molecular mechanisms and specific target protein remain unknown. Here, we found that 7,3',4'-THIF, but not daidzein, inhibited α-melanocyte-stimulating hormone (MSH)-induced intracellular and extracellular melanin production in B16F10 cells by directly targeting melanocortin 1 receptor (MC1R). Western blot data showed that 7,3',4'-THIF inhibited α-MSH-induced tyrosinase, tyrosinase-related protein-1 (TYRP-1), and tyrosinase-related protein-2 (TYRP-2) expressions through the inhibition of Microphthalmia-associated transcription factor (MITF) expression and cAMP response element-binding (CREB) phosphorylation. 7,3',4'-THIF also inhibited α-MSH-induced dephosphorylation of AKT and phosphorylation of p38 and cAMP-dependent protein kinase (PKA). cAMP and Pull-down assays indicated that 7,3',4'-THIF strongly inhibited forskolin-induced intracellular cAMP production and bound MC1R directly by competing with α-MSH. Moreover, 7,3',4'-THIF inhibited α-MSH-induced intracellular melanin production in human epidermal melanocytes (HEMs). Collectively, these results demonstrate that 7,3',4'-THIF targets MC1R, resulting in the suppression of melanin production, suggesting a protective role for 7,3',4'-THIF against melanogenesis.
Keywords: 3′; 4′-Trihydroxyisoflavone; 7; depigmentation; melanocortin 1 receptor; melanogenesis; α-MSH.
Copyright © 2020 Kim, Lee, Kim, Yeom, Park, di Luccio, Chen, Dong, Lee and Kang.
Conflict of interest statement
MY and JP were employed by company Amorepacific Corporation R&D Center. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Sesamol Inhibited Melanogenesis by Regulating Melanin-Related Signal Transduction in B16F10 Cells.Int J Mol Sci. 2018 Apr 7;19(4):1108. doi: 10.3390/ijms19041108. Int J Mol Sci. 2018. PMID: 29642438 Free PMC article.
-
7,3',4'-Trihydroxyisoflavone, a metabolite of the soy isoflavone daidzein, suppresses ultraviolet B-induced skin cancer by targeting Cot and MKK4.J Biol Chem. 2011 Apr 22;286(16):14246-56. doi: 10.1074/jbc.M110.147348. Epub 2011 Mar 4. J Biol Chem. 2011. PMID: 21378167 Free PMC article.
-
Aqueous fraction from Cuscuta japonica seed suppresses melanin synthesis through inhibition of the p38 mitogen-activated protein kinase signaling pathway in B16F10 cells.J Ethnopharmacol. 2012 May 7;141(1):338-44. doi: 10.1016/j.jep.2012.02.043. Epub 2012 Mar 3. J Ethnopharmacol. 2012. PMID: 22414478
-
The melanocortin-1 receptor and human pigmentation.Ann N Y Acad Sci. 1999 Oct 20;885:117-33. doi: 10.1111/j.1749-6632.1999.tb08669.x. Ann N Y Acad Sci. 1999. PMID: 10816645 Review.
-
Significance of the melanocortin 1 receptor in regulating human melanocyte pigmentation, proliferation, and survival.Ann N Y Acad Sci. 2003 Jun;994:359-65. doi: 10.1111/j.1749-6632.2003.tb03200.x. Ann N Y Acad Sci. 2003. PMID: 12851336 Review.
Cited by
-
Anti-Melanogenic and Antioxidant Effects of Cell-Free Supernatant from Lactobacillus gasseri BNR17.Microorganisms. 2022 Apr 8;10(4):788. doi: 10.3390/microorganisms10040788. Microorganisms. 2022. PMID: 35456838 Free PMC article.
-
In Vitro Human Monoamine Oxidase Inhibition and Human Dopamine D4 Receptor Antagonist Effect of Natural Flavonoids for Neuroprotection.Int J Mol Sci. 2023 Nov 1;24(21):15859. doi: 10.3390/ijms242115859. Int J Mol Sci. 2023. PMID: 37958841 Free PMC article.
-
Biochemical Mechanism of Thai Fermented Soybean Extract on UVB-Induced Skin Keratinocyte Damage and Inflammation.Int J Mol Sci. 2025 Apr 5;26(7):3418. doi: 10.3390/ijms26073418. Int J Mol Sci. 2025. PMID: 40244292 Free PMC article.
-
Biocompatible exosomes derived from Pinctada martensii mucus for therapeutic melanin regulation via α-MSH/NF-κB/MITF pathway.Regen Biomater. 2025 Jul 3;12:rbaf072. doi: 10.1093/rb/rbaf072. eCollection 2025. Regen Biomater. 2025. PMID: 40747328 Free PMC article.
-
Protective Function of Malus baccata (L.) Borkh Methanol Extract against UVB/Hydrogen Peroxide-Induced Skin Aging via Inhibition of MAPK and NF-κB Signaling.Plants (Basel). 2022 Sep 11;11(18):2368. doi: 10.3390/plants11182368. Plants (Basel). 2022. PMID: 36145769 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials

