Multimodality Imaging for Discordant Low-Gradient Aortic Stenosis: Assessing the Valve and the Myocardium
- PMID: 33344514
- PMCID: PMC7744378
- DOI: 10.3389/fcvm.2020.570689
Multimodality Imaging for Discordant Low-Gradient Aortic Stenosis: Assessing the Valve and the Myocardium
Abstract
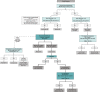
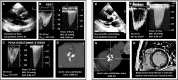
Aortic stenosis (AS) is a disease of the valve and the myocardium. A correct assessment of the valve disease severity is key to define the need for aortic valve replacement (AVR), but a better understanding of the myocardial consequences of the increased afterload is paramount to optimize the timing of the intervention. Transthoracic echocardiography remains the cornerstone of AS assessment, as it is universally available, and it allows a comprehensive structural and hemodynamic evaluation of both the aortic valve and the rest of the heart. However, it may not be sufficient as a significant proportion of patients with severe AS presents with discordant grading (i.e., an AVA ≤ 1 cm2 and a mean gradient <40 mmHg) which raises uncertainty about the true severity of AS and the need for AVR. Several imaging modalities (transesophageal or stress echocardiography, computed tomography, cardiovascular magnetic resonance, positron emission tomography) exist that allow a detailed assessment of the stenotic aortic valve and the myocardial remodeling response. This review aims to provide an updated overview of these multimodality imaging techniques and seeks to highlight a practical approach to help clinical decision making in the challenging group of patients with discordant low-gradient AS.
Keywords: aortic stenosis; computed tomography; echocardiography; low-gradient aortic stenosis; magnetic resonance imaging.
Copyright © 2020 Guzzetti, Annabi, Pibarot and Clavel.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Discordant Grading of Aortic Stenosis Severity: Echocardiographic Predictors of Survival Benefit Associated With Aortic Valve Replacement.JACC Cardiovasc Imaging. 2016 Jul;9(7):797-805. doi: 10.1016/j.jcmg.2015.09.026. Epub 2016 May 18. JACC Cardiovasc Imaging. 2016. PMID: 27209111
-
Cardiac Imaging for Assessing Low-Gradient Severe Aortic Stenosis.JACC Cardiovasc Imaging. 2017 Feb;10(2):185-202. doi: 10.1016/j.jcmg.2017.01.002. JACC Cardiovasc Imaging. 2017. PMID: 28183438 Review.
-
The complex nature of discordant severe calcified aortic valve disease grading: new insights from combined Doppler echocardiographic and computed tomographic study.J Am Coll Cardiol. 2013 Dec 17;62(24):2329-38. doi: 10.1016/j.jacc.2013.08.1621. Epub 2013 Sep 24. J Am Coll Cardiol. 2013. PMID: 24076528
-
Postoperative Reverse Remodeling and Symptomatic Improvement in Normal-Flow Low-Gradient Aortic Stenosis After Aortic Valve Replacement.Circ Cardiovasc Imaging. 2017 Dec;10(12):e006580. doi: 10.1161/CIRCIMAGING.117.006580. Circ Cardiovasc Imaging. 2017. PMID: 29222121
-
Imaging for Predicting and Assessing Prosthesis-Patient Mismatch After Aortic Valve Replacement.JACC Cardiovasc Imaging. 2019 Jan;12(1):149-162. doi: 10.1016/j.jcmg.2018.10.020. JACC Cardiovasc Imaging. 2019. PMID: 30621987 Review.
Cited by
-
Aliased Flow Signal Planimetry by Cardiovascular Magnetic Resonance Imaging for Grading Aortic Stenosis Severity: A Prospective Pilot Study.Front Cardiovasc Med. 2021 Oct 18;8:752340. doi: 10.3389/fcvm.2021.752340. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 34733896 Free PMC article.
-
Peak flow measurements in patients with severe aortic stenosis: a prospective comparative study between cardiovascular magnetic resonance 2D and 4D flow and transthoracic echocardiography.J Cardiovasc Magn Reson. 2021 Nov 15;23(1):132. doi: 10.1186/s12968-021-00825-1. J Cardiovasc Magn Reson. 2021. PMID: 34775954 Free PMC article.
-
Harnessing feature extraction capacities from a pre-trained convolutional neural network (VGG-16) for the unsupervised distinction of aortic outflow velocity profiles in patients with severe aortic stenosis.Eur Heart J Digit Health. 2022 Apr 22;3(2):153-168. doi: 10.1093/ehjdh/ztac004. eCollection 2022 Jun. Eur Heart J Digit Health. 2022. PMID: 36713009 Free PMC article.
-
Aortic Valve Calcium Score: Applications in Clinical Practice and Scientific Research-A Narrative Review.J Clin Med. 2024 Jul 11;13(14):4064. doi: 10.3390/jcm13144064. J Clin Med. 2024. PMID: 39064103 Free PMC article. Review.
References
-
- Berthelot-Richer M, Pibarot P, Capoulade R, Dumesnil JG, Dahou A, Thébault C, et al. . Discordant grading of aortic stenosis severity: echocardiographic predictors of survival benefit associated with aortic valve replacement. JACC Cardiovasc Imaging. (2016) 9:797–805. 10.1016/j.jcmg.2015.09.026 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

