The Suppression of Th1 Response by Inducing TGF-β1 From Regulatory T Cells in Bovine Mycoplasmosis
- PMID: 33344537
- PMCID: PMC7738317
- DOI: 10.3389/fvets.2020.609443
The Suppression of Th1 Response by Inducing TGF-β1 From Regulatory T Cells in Bovine Mycoplasmosis
Abstract
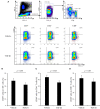
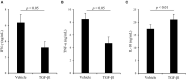
Regulatory T cells (Tregs) regulate immune responses and maintain host immune homeostasis. Tregs contribute to the disease progression of several chronic infections by oversuppressing immune responses via the secretion of immunosuppressive cytokines, such as transforming growth factor (TGF)-β and interleukin-10. In the present study, we examined the association of Tregs with Mycoplasma bovis infection, in which immunosuppression is frequently observed. Compared with uninfected cattle, the percentage of Tregs, CD4+CD25highFoxp3+ T cells, was increased in M. bovis-infected cattle. Additionally, the plasma of M. bovis-infected cattle contained the high concentrations of TGF-β1, and M. bovis infection induced TGF-β1 production from bovine immune cells in in vitro cultures. Finally, we analyzed the immunosuppressive effects of TGF-β1 on bovine immune cells. Treatment with TGF-β1 significantly decreased the expression of CD69, an activation marker, in T cells, and Th1 cytokine production in vitro. These results suggest that the increase in Tregs and TGF-β1 secretion could be one of the immunosuppressive mechanisms and that lead to increased susceptibility to other infections in terms of exacerbation of disease during M. bovis infection.
Keywords: Mycoplasma bovis; TGF-β1; cattle; immunosuppression; regulatory T cell.
Copyright © 2020 Sajiki, Konnai, Goto, Okagawa, Ohira, Shimakura, Maekawa, Gondaira, Higuchi, Tajima, Hirano, Kohara, Murata and Ohashi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources
Research Materials

