Evaluating the Alzheimer's disease data landscape
- PMID: 33344750
- PMCID: PMC7744022
- DOI: 10.1002/trc2.12102
Evaluating the Alzheimer's disease data landscape
Abstract
Introduction: Numerous studies have collected Alzheimer's disease (AD) cohort data sets. To achieve reproducible, robust results in data-driven approaches, an evaluation of the present data landscape is vital.
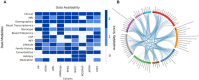
Methods: Previous efforts relied exclusively on metadata and literature. Here, we evaluate the data landscape by directly investigating nine patient-level data sets generated in major clinical cohort studies.
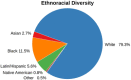
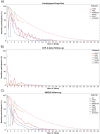
Results: The investigated cohorts differ in key characteristics, such as demographics and distributions of AD biomarkers. Analyzing the ethnoracial diversity revealed a strong bias toward White/Caucasian individuals. We described and compared the measured data modalities. Finally, the available longitudinal data for important AD biomarkers was evaluated. All results are explorable through our web application ADataViewer (https://adata.scai.fraunhofer.de).
Discussion: Our evaluation exposed critical limitations in the AD data landscape that impede comparative approaches across multiple data sets. Comparison of our results to those gained by metadata-based approaches highlights that thorough investigation of real patient-level data is imperative to assess a data landscape.
Keywords: Alzheimer's disease; FAIR data; biomarker; clinical study; cohort; cohort study; data; data access; data set; data sharing; data viewer; data‐driven; dementia; disease modeling; magnetic resonance imaging; open‐science; patient level data.
© 2020 The Authors. Alzheimer's & Dementia: Translational Research & Clinical Interventions published by Wiley Periodicals, Inc. on behalf of Alzheimer's Association.
Figures
References
-
- Kalra D. The importance of real‐world data to precision medicine. Per Med. 2019;16(2):79‐82. - PubMed
-
- Ferreira D, Hansson O, Barroso J, et al. The interactive effect of demographic and clinical factors on hippocampal volume: a multicohort study on 1958 cognitively normal individuals. Hippocampus. 2017;27(6):653‐667. - PubMed
Grants and funding
- P50 AG005142/AG/NIA NIH HHS/United States
- P30 AG010133/AG/NIA NIH HHS/United States
- P50 AG005146/AG/NIA NIH HHS/United States
- P50 AG047266/AG/NIA NIH HHS/United States
- P30 AG008017/AG/NIA NIH HHS/United States
- P30 AG010161/AG/NIA NIH HHS/United States
- P50 AG025688/AG/NIA NIH HHS/United States
- P50 AG005133/AG/NIA NIH HHS/United States
- P50 AG005138/AG/NIA NIH HHS/United States
- P50 AG047366/AG/NIA NIH HHS/United States
- P30 AG019610/AG/NIA NIH HHS/United States
- P30 AG028383/AG/NIA NIH HHS/United States
- R01 AG015819/AG/NIA NIH HHS/United States
- P30 AG013854/AG/NIA NIH HHS/United States
- P30 AG053760/AG/NIA NIH HHS/United States
- P30 AG066444/AG/NIA NIH HHS/United States
- P30 AG062428/AG/NIA NIH HHS/United States
- P30 AG010124/AG/NIA NIH HHS/United States
- P50 AG023501/AG/NIA NIH HHS/United States
- P30 AG062421/AG/NIA NIH HHS/United States
- U01 AG024904/AG/NIA NIH HHS/United States
- P30 AG035982/AG/NIA NIH HHS/United States
- P50 AG008702/AG/NIA NIH HHS/United States
- U01 AG016976/AG/NIA NIH HHS/United States
- P30 AG008051/AG/NIA NIH HHS/United States
- P50 AG005681/AG/NIA NIH HHS/United States
- P30 AG013846/AG/NIA NIH HHS/United States
- R01 AG017917/AG/NIA NIH HHS/United States
- P50 AG047270/AG/NIA NIH HHS/United States
- P30 AG062429/AG/NIA NIH HHS/United States
- P50 AG005136/AG/NIA NIH HHS/United States
- P30 AG049638/AG/NIA NIH HHS/United States
- P30 AG012300/AG/NIA NIH HHS/United States
- P30 AG062422/AG/NIA NIH HHS/United States
- P50 AG016573/AG/NIA NIH HHS/United States
- P30 AG062715/AG/NIA NIH HHS/United States
- P30 AG010129/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
