A paradox: Fe2+-containing agents decreased ROS and apoptosis induced by CoNPs in vascular endothelial cells by inhibiting HIF-1α
- PMID: 33345265
- PMCID: PMC7796189
- DOI: 10.1042/BSR20203456
A paradox: Fe2+-containing agents decreased ROS and apoptosis induced by CoNPs in vascular endothelial cells by inhibiting HIF-1α
Abstract
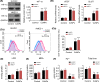
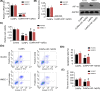
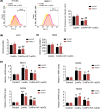
Cobalt nanoparticles (CoNPs) released from hip joint implants are known to have a toxic effect on several organs probably through increasing reactive oxygen species (ROS). Ferrous ion (Fe2+) is well-known to enhance oxidative stress by catalysing the production of ROS. However, in our pilot study, we found that Fe2+ conversely inhibited the ROS production induced by CoNPs. To elucidate the underlying mechanism, the present study treated vascular endothelial HUVEC and HMEC-1 cells with CoNPs alone or in combination with ferrous lactate [Fe(CH3CHOHCOO)2], ferrous succinate [Fe(CH2COO)2], and ferrous chloride (FeCl2). CoNP toxicity was evaluated by measuring cell viability, rate of apoptosis and lactose dehydrogenase (LDH) release, and intracellular ROS levels. Treatment with CoNPs decreased cell viability, LDH release, and ROS production and increased apoptosis. CoNPs increased hypoxia-inducible factor-1α (HIF-1α) protein level and mRNA levels of vascular endothelial growth factor (VEGF) and glucose transporter 1 (GLUT1) downstream of HIF-1α signalling. Silencing HIF-1α attenuated CoNP toxicity, as seen by recovery of cell viability, LDH release, and ROS levels and reduced apoptosis. CoNPs caused a pronounced reduction of Fe2+ in cells, but supplementation with Fe(CH3CHOHCOO)2, Fe(CH2COO)2, and FeCl2 restored Fe2+ levels and inhibited HIF-1α activation. Moreover, all three Fe2+-containing agents conferred protection from CoNPs; Fe(CH3CHOHCOO)2 and Fe(CH2COO)2 more effectively than FeCl2. In summary, the present study revealed that CoNPs exert their toxicity on human vascular endothelial cells by depleting intracellular Fe2+ level, which causes activation of HIF-1α signalling. Supplements of Fe2+, especially in the form of Fe(CH3CHOHCOO)2 and Fe(CH2COO)2, mitigated CoNP toxicity.
Keywords: CoNPs; Fe2+-containing agents; hypoxia-inducible factor; vascular endothelial cells.
© 2021 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures





Similar articles
-
Supplementation with Fe2+-containing agents mitigated the toxic effect of CoNPs in vascular endothelial cells by inhibiting activation of hypoxia-inducible factor.Biosci Rep. 2020 Aug 3:BSR20201260. doi: 10.1042/BSR20201260. Online ahead of print. Biosci Rep. 2020. PMID: 32744328
-
N-acetylcysteine Attenuates Cobalt Nanoparticle-Induced Cytotoxic Effects through Inhibition of Cell Death, Reactive Oxygen Species-related Signaling and Cytokines Expression.Orthop Surg. 2016 Nov;8(4):496-502. doi: 10.1111/os.12298. Orthop Surg. 2016. PMID: 28032714 Free PMC article.
-
Acute Methylmercury Exposure and the Hypoxia-Inducible Signaling Pathway under Normoxic Conditions in the Rat Brain and Astrocytes in Vitro.Environ Health Perspect. 2019 Dec;127(12):127006. doi: 10.1289/EHP5139. Epub 2019 Dec 18. Environ Health Perspect. 2019. PMID: 31850806 Free PMC article.
-
Causation by Diesel Exhaust Particles of Endothelial Dysfunctions in Cytotoxicity, Pro-inflammation, Permeability, and Apoptosis Induced by ROS Generation.Cardiovasc Toxicol. 2017 Oct;17(4):384-392. doi: 10.1007/s12012-016-9364-0. Cardiovasc Toxicol. 2017. PMID: 26965709 Review.
-
Research progress on toxicity, function, and mechanism of metal oxide nanoparticles on vascular endothelial cells.J Appl Toxicol. 2021 May;41(5):683-700. doi: 10.1002/jat.4121. Epub 2020 Nov 26. J Appl Toxicol. 2021. PMID: 33244813 Review.
Cited by
-
The association of Serum Klotho with the prevalence of cardiovascular disease and prognosis in general population: results from the National Health and Nutrition Examination Survey 2007-2016.J Geriatr Cardiol. 2024 Nov 28;21(11):1034-1046. doi: 10.26599/1671-5411.2024.11.008. J Geriatr Cardiol. 2024. PMID: 39734651 Free PMC article.
-
Fluorescent Carbon Dots for Sensitive and Rapid Monitoring of Intracellular Ferrous Ion.Biosensors (Basel). 2022 Jan 14;12(1):41. doi: 10.3390/bios12010041. Biosensors (Basel). 2022. PMID: 35049669 Free PMC article.
-
Transition metals in angiogenesis - A narrative review.Mater Today Bio. 2023 Aug 3;22:100757. doi: 10.1016/j.mtbio.2023.100757. eCollection 2023 Oct. Mater Today Bio. 2023. PMID: 37593220 Free PMC article. Review.
-
Association between metallic implants and stroke in US adults from NHANES 2015-2023 a cross-sectional study.Front Aging Neurosci. 2024 Dec 20;16:1505645. doi: 10.3389/fnagi.2024.1505645. eCollection 2024. Front Aging Neurosci. 2024. PMID: 39759400 Free PMC article.
-
Exploring the Causal Effects of Mineral Metabolism Disorders on Telomere and Mitochondrial DNA: A Bidirectional Two-Sample Mendelian Randomization Analysis.Nutrients. 2024 May 8;16(10):1417. doi: 10.3390/nu16101417. Nutrients. 2024. PMID: 38794655 Free PMC article.
References
-
- Laovitthayanggoon S., Henderson C.J., McCluskey C., MacDonald M., Tate R.J., Grant M.H. et al. . (2019) Cobalt administration causes reduced contractility with parallel increases in TRPC6 and TRPM7 transporter protein expression in adult rat hearts. Cardiovasc. Toxicol. 19, 276–286 10.1007/s12012-018-9498-3 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

