Brain mechanisms of HPA axis regulation: neurocircuitry and feedback in context Richard Kvetnansky lecture
- PMID: 33345670
- PMCID: PMC8034599
- DOI: 10.1080/10253890.2020.1859475
Brain mechanisms of HPA axis regulation: neurocircuitry and feedback in context Richard Kvetnansky lecture
Abstract
Regulation of stress reactivity is a fundamental priority of all organisms. Stress responses are critical for survival, yet can also cause physical and psychological damage. This review provides a synopsis of brain mechanisms designed to control physiological responses to stress, focusing primarily on glucocorticoid secretion via the hypothalamo-pituitary-adrenocortical (HPA) axis. The literature provides strong support for multi-faceted control of HPA axis responses, involving both direct and indirect actions at paraventricular nucleus (PVN) corticotropin releasing hormone neurons driving the secretory cascade. The PVN is directly excited by afferents from brainstem and hypothalamic circuits, likely relaying information on homeostatic challenge. Amygdala subnuclei drive HPA axis responses indirectly via disinhibition, mediated by GABAergic relays onto PVN-projecting neurons in the hypothalamus and bed nucleus of the stria terminalis (BST). Inhibition of stressor-evoked HPA axis responses is mediated by an elaborate network of glucocorticoid receptor (GR)-containing circuits, providing a distributed negative feedback signal that inhibits PVN neurons. Prefrontal and hippocampal neurons play a major role in HPA axis inhibition, again mediated by hypothalamic and BST GABAergic relays to the PVN. The complexity of the regulatory process suggests that information on stressors is integrated across functional disparate brain circuits prior to accessing the PVN, with regions such as the BST in prime position to relay contextual information provided by these sources into appropriate HPA activation. Dysregulation of the HPA in disease is likely a product of inappropriate checks and balances between excitatory and inhibitory inputs ultimately impacting PVN output.
Keywords: HPA Axis; amygdala; bed nucleus of the stria terminalis; glucocorticoids; hippocampus; prefrontal cortex.
Conflict of interest statement
Declaration of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
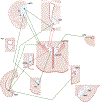
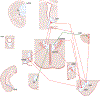
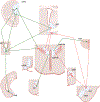
References
-
- Ahima RS, Krozowski Z, Harlan RE, 1991. Type I corticosteroid receptor-like immunoreactivity in the rat CNS: Distribution and Regulation by Corticosteroids. J. Comp. Neurol 313, 522–538. - PubMed
-
- Allen, 2007. ALLEN Mouse Brain Atlas. Gene Expr.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
