Negative pressure increases microvascular perfusion during severe hemorrhagic shock
- PMID: 33346023
- PMCID: PMC7856175
- DOI: 10.1016/j.mvr.2020.104125
Negative pressure increases microvascular perfusion during severe hemorrhagic shock
Abstract
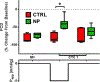
Hemorrhagic shock (HS) is a severe life-threatening condition characterized by loss of blood volume and a lack of oxygen (O2) delivery to tissues. The objective of this study was to examine the impact of manipulating Starling forces in the microcirculation during HS to increase microvascular perfusion without restoring blood volume or increasing O2 carrying capacity. To decrease interstitial tissue pressure, we developed a non-contact system to locally apply negative pressure and manipulate the pressure balance in capillaries, while allowing for visualization of the microcirculation. Golden Syrian hamsters were instrumented with dorsal window chambers and subjected to a controlled hemorrhaged of 50% of the animal's blood volume without any fluid resuscitation. A negative pressure chamber was attached to the dorsal window chamber and a constant negative pressure was applied. Hemodynamic parameters (including microvascular diameter, blood flow, and functional capillary density [FCD]) were measured before and during the four hours following the hemorrhage, with and without applied negative pressure. Blood flow significantly increased in arterioles during negative pressure. The increase in flow through arterioles also improved microvascular perfusion as reflected by increased FCD. These results indicate that negative pressure increases flow in the microcirculation when fluid resuscitation is not available, thus restoring blood flow, oxygen delivery, and preventing the accumulation of metabolic waste. Applying negative pressure might allow for control of microvascular blood flow and oxygen delivery to specific tissue areas.
Keywords: Intravital microscopy; Reabsorption; Starling forces; Tissue perfusion.
Copyright © 2020 Elsevier Inc. All rights reserved.
Figures
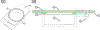



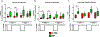
References
-
- American College of Surgeons and Committee on Trauma, Advanced trauma life support: student course manual. Chicago, IL: American College of Surgeons, 2018.
-
- Pappano AJ, Wier WG, and Levy MN, Cardiovascular physiology, 10th ed Philadelphia, PA: Elsevier/Mosby, 2013.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

