Epidermal Acyl-CoA-binding protein is indispensable for systemic energy homeostasis
- PMID: 33346070
- PMCID: PMC7797911
- DOI: 10.1016/j.molmet.2020.101144
Epidermal Acyl-CoA-binding protein is indispensable for systemic energy homeostasis
Abstract
Objectives: The skin is the largest sensory organ of the human body and plays a fundamental role in regulating body temperature. However, adaptive alterations in skin functions and morphology have only vaguely been associated with physiological responses to cold stress or sensation of ambient temperatures. We previously found that loss of acyl-CoA-binding protein (ACBP) in keratinocytes upregulates lipolysis in white adipose tissue and alters hepatic lipid metabolism, suggesting a link between epidermal barrier functions and systemic energy metabolism.
Methods: To assess the physiological responses to loss of ACBP in keratinocytes in detail, we used full-body ACBP-/- and skin-specific ACBP-/- knockout mice to clarify how loss of ACBP affects 1) energy expenditure by indirect calorimetry, 2) response to high-fat feeding and a high oral glucose load, and 3) expression of brown-selective gene programs by quantitative PCR in inguinal WAT (iWAT). To further elucidate the role of the epidermal barrier in systemic energy metabolism, we included mice with defects in skin structural proteins (ma/ma Flgft/ft) in these studies.
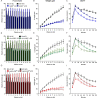
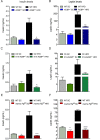
Results: We show that the ACBP-/- mice and skin-specific ACBP-/- knockout mice exhibited increased energy expenditure, increased food intake, browning of the iWAT, and resistance to diet-induced obesity. The metabolic phenotype, including browning of the iWAT, was reversed by housing the mice at thermoneutrality (30 °C) or pharmacological β-adrenergic blocking. Interestingly, these findings were phenocopied in flaky tail mice (ma/ma Flgft/ft). Taken together, we demonstrate that a compromised epidermal barrier induces a β-adrenergic response that increases energy expenditure and browning of the white adipose tissue to maintain a normal body temperature.
Conclusions: Our findings show that the epidermal barrier plays a key role in maintaining systemic metabolic homeostasis. Thus, regulation of epidermal barrier functions warrants further attention to understand the regulation of systemic metabolism in further detail.
Keywords: Acyl-CoA binding protein; Adipose tissue; Browning; Diet induced obesity; Energy expenditure; Epidermal barrier; Filaggrin; β-adrenergic signaling.
Copyright © 2020 The Author(s). Published by Elsevier GmbH.. All rights reserved.
Figures
Similar articles
-
Acyl CoA-binding protein in brown adipose tissue acts as a negative regulator of adaptive thermogenesis.Mol Metab. 2025 Jun;96:102153. doi: 10.1016/j.molmet.2025.102153. Epub 2025 Apr 11. Mol Metab. 2025. PMID: 40220929 Free PMC article.
-
Delayed hepatic adaptation to weaning in ACBP-/- mice is caused by disruption of the epidermal barrier.Cell Rep. 2013 Dec 12;5(5):1403-12. doi: 10.1016/j.celrep.2013.11.010. Epub 2013 Dec 5. Cell Rep. 2013. PMID: 24316079
-
Housing temperature affects the acute and chronic metabolic adaptations to exercise in mice.J Physiol. 2019 Sep;597(17):4581-4600. doi: 10.1113/JP278221. Epub 2019 Jul 11. J Physiol. 2019. PMID: 31297830
-
CIDE Family-Mediated Unique Lipid Droplet Morphology in White Adipose Tissue and Brown Adipose Tissue Determines the Adipocyte Energy Metabolism.J Atheroscler Thromb. 2017 Oct 1;24(10):989-998. doi: 10.5551/jat.RV17011. Epub 2017 Sep 5. J Atheroscler Thromb. 2017. PMID: 28883211 Free PMC article. Review.
-
P2X7 Receptor in the Management of Energy Homeostasis: Implications for Obesity, Dyslipidemia, and Insulin Resistance.Front Endocrinol (Lausanne). 2020 May 12;11:199. doi: 10.3389/fendo.2020.00199. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 32528404 Free PMC article. Review.
Cited by
-
Contrasting recruitment of skin-associated adipose depots during cold challenge of mouse and human.J Physiol. 2022 Feb;600(4):847-868. doi: 10.1113/JP280922. Epub 2021 Apr 15. J Physiol. 2022. PMID: 33724479 Free PMC article.
-
Acyl CoA-binding protein in brown adipose tissue acts as a negative regulator of adaptive thermogenesis.Mol Metab. 2025 Jun;96:102153. doi: 10.1016/j.molmet.2025.102153. Epub 2025 Apr 11. Mol Metab. 2025. PMID: 40220929 Free PMC article.
-
Effects of Semaglutide on Cardiac Protein Expression and Cardiac Function of Obese Mice.J Inflamm Res. 2022 Nov 24;15:6409-6425. doi: 10.2147/JIR.S391859. eCollection 2022. J Inflamm Res. 2022. PMID: 36452054 Free PMC article.
-
Effects of acyl-coenzyme A binding protein (ACBP)/diazepam-binding inhibitor (DBI) on body mass index.Cell Death Dis. 2021 Jun 9;12(6):599. doi: 10.1038/s41419-021-03864-9. Cell Death Dis. 2021. PMID: 34108446 Free PMC article.
-
Brown Fat Anatomy in Humans and Rodents.Methods Mol Biol. 2022;2448:19-42. doi: 10.1007/978-1-0716-2087-8_2. Methods Mol Biol. 2022. PMID: 35167088
References
-
- Feingold K.R., Denda M. Regulation of permeability barrier homeostasis. Clinical Dermatology. 2012;30(3):263–268. - PubMed
-
- Kruse V., Neess D., Faergeman N.J. The significance of epidermal lipid metabolism in whole-body physiology. Trends in Endocrinology and Metabolism. 2017;28(9):669–683. - PubMed
-
- Sampath H., Flowers M.T., Liu X., Paton C.M., Sullivan R., Chu K. Skin-specific deletion of steaCold Spring Harbor Perspectives in Medicineroyl-CoA desaturase-1 alters skin lipid composition and protects mice from high fat diet-induced obesity. Journal of Biological Chemistry. 2009;284(30):19961–19973. - PMC - PubMed
-
- Westerberg R., Tvrdik P., Unden A.B., Mansson J.E., Norlen L., Jakobsson A. Role for ELOVL3 and fatty acid chain length in development of hair and skin function. Journal of Biological Chemistry. 2004;279(7):5621–5629. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

