Novel SOD1 monoclonal antibodies against the electrostatic loop preferentially detect misfolded SOD1 aggregates
- PMID: 33346076
- PMCID: PMC9171709
- DOI: 10.1016/j.neulet.2020.135553
Novel SOD1 monoclonal antibodies against the electrostatic loop preferentially detect misfolded SOD1 aggregates
Abstract
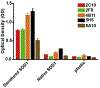
Amyotrophic lateral sclerosis (ALS) is a progressive neurological disease that leads to motor neuron degeneration and paralysis. Superoxide dismutase (SOD1) mutations are the second most common cause of familial ALS and are responsible for up to 20 % of familial ALS cases. In ALS patients, SOD1 can form toxic misfolded aggregates that deposit in the brain and spinal cord. To better detect SOD1 aggregates and expand the repertoire of conformational SOD1 antibodies, SOD1 monoclonal antibodies were generated by immunizing SOD1 knockout mice with an SOD1 fragment consisting of amino acids 129-146, which make up part of the electrostatic loop. A series of hybridomas secreting antibodies were screened and five different SOD1 monoclonal antibodies (2C10, 2F8, 4B11, 5H5, and 5A10) were found to preferentially detect denatured or aggregated SOD1 by enzyme-linked immunosorbent assay (ELISA), filter trap assay, and immunohistochemical analysis in SOD1 mouse models. The staining with these antibodies was compared to Campbell-Switzer argyrophilic reactivity of pathological inclusions. These new conformational selective SOD1 antibodies will be useful for clinical diagnosis of SOD1 ALS and potentially for passive immunotherapy.
Keywords: Amyotrophic lateral sclerosis; Antibodies; Frontotemporal dementia; Pathology; Silver stain; Superoxide dismutase 1.
Copyright © 2020 Elsevier B.V. All rights reserved.
Conflict of interest statement
Figures





References
-
- Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O’Regan JP, Deng H-X, Rahmani Z, Krizus A, McKenna-Yasek D, Cayabyab A, Gaston SM, Berger R, Tanzi RE, Halperin JJ, Herzfeldt B, Van den Bergh R, Hung W-Y, Bird T, Deng G, Mulder DW, Smyth C, Laing NG, Soriano E, Pericak–Vance MA, Haines J, Rouleau GA, Gusella JS, Horvitz HR, Brown RH, Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis, Nature 362 (1993) 59–62. 10.1038/362059a0. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

