Mitigation of Blood Borne Cell Attachment to Metal Implants through CD47-Derived Peptide Immobilization
- PMID: 33346187
- PMCID: PMC8764910
- DOI: 10.3791/61545
Mitigation of Blood Borne Cell Attachment to Metal Implants through CD47-Derived Peptide Immobilization
Abstract
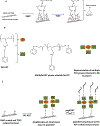
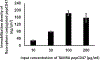
The key complications associated with bare metal stents and drug eluting stents are in-stent restenosis and late stent thrombosis, respectively. Thus, improving the biocompatibility of metal stents remains a significant challenge. The goal of this protocol is to describe a robust technique of metal surface modification by biologically active peptides to increase biocompatibility of blood contacting medical implants, including endovascular stents. CD47 is an immunological species-specific marker of self and has anti-inflammatory properties. Studies have shown that a 22 amino acid peptide corresponding to the Ig domain of CD47 in the extracellular region (pepCD47), has anti-inflammatory properties like the full-length protein. In vivo studies in rats, and ex vivo studies in rabbit and human blood experimental systems from our lab have demonstrated that pepCD47 immobilization on metals improves their biocompatibility by preventing inflammatory cell attachment and activation. This paper describes the step-by step protocol for the functionalization of metal surfaces and peptide attachment. The metal surfaces are modified using polyallylamine bisphosphate with latent thiol groups (PABT) followed by deprotection of thiols and amplification of thiol-reactive sites via reaction with polyethyleneimine installed with pyridyldithio groups (PEI-PDT). Finally, pepCD47, incorporating terminal cysteine residues connected to the core peptide sequence through a dual 8-amino-3,6-dioxa-octanoyl spacer, are attached to the metal surface via disulfide bonds. This methodology of peptide attachment to metal surface is efficient and relatively inexpensive and thus can be applied to improve biocompatibility of several metallic biomaterials.
Figures



References
-
- Iqbal J, Gunn J & Serruys PW Coronary stents: historical development, current status and future directions. British Medical Bulletin. 106 193–211, (2013). - PubMed
-
- Hoffmann R et al. Patterns and mechanisms of in-stent restenosis. A serial intravascular ultrasound study. Circulation. 94 (6), 1247–1254, (1996). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
