The inhibitory effect of TU-100 on hepatic stellate cell activation in the tumor microenvironment
- PMID: 33346211
- PMCID: PMC7733620
- DOI: 10.18632/oncotarget.27835
The inhibitory effect of TU-100 on hepatic stellate cell activation in the tumor microenvironment
Abstract
Introduction: The tumor microenvironment is involved in acquiring tumor malignancies of colorectal liver metastasis (CRLM). We have reported that TU-100 (Daikenchuto) suppresses hepatic stellate cell (HSC) activation in obstructive jaundice. In this study, we report new findings as the direct and indirect inhibitory effects of TU-100 on cancer cell growth through the suppression of HSC activation.
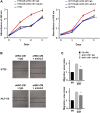
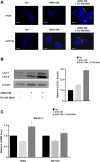
Materials and methods: The HSCs (LX2) were cultured in colon cancer cells (HCT116 and HT29)-conditioned medium (CM) with or without TU-100 treatment (90, 270, 900 μg/ml). Activated HSCs (aHSCs) were detected by α-SMA and IL-6 mRNA expressions and cytokine arrays of HSC's culture supernatants. Cancer cell growth was analyzed for proliferation and migration ability, compared with TU-100 treatment. To investigate the direct anti-tumor effect of TU-100, cancer cells were cultured in the presence of aHSC-CM and TU-100 (90, 270, 900) or aHSC-CM alone, and assessed autophagosomes, conversion to LC3-II protein, and Beclin-1 mRNA expression.
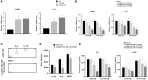
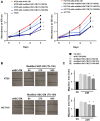
Results: Colon cancer-CM significantly increased α-SMA and IL-6 mRNA expressions of aHSC. α-SMA and IL-6 mRNA expressions of aHSC, and IL-6 secretions from aHSCs were significantly decreased with TU-100 (270, 900) treatment, compared to colon cancer-CM alone. Compared with normal culture medium, aHSC-CM led to a significantly increased cell number and modified HSC-CM (TU-100; 270, 900) significantly suppressed cancer cell growth and migration. TU-100 (900) treatment induced autophagy and significantly promoted the autophagic cell death.
Conclusions: TU-100 inhibited colon cancer cell malignant potential by both suppressing HSC activation and inducing directly autophagy of cancer cells.
Keywords: CRC; CRLM; HSC; IL-6; TU-100.
Copyright: © 2020 Wada et al.
Conflict of interest statement
CONFLICTS OF INTEREST We state any potential conflicts of interest regarding our study as follows; Mitsuo Shimada received grant support from Tsumura & Co. The other authors declare no conflicts of interest in association with the present study.
Figures

Similar articles
-
BMI-1 activates hepatic stellate cells to promote the epithelial-mesenchymal transition of colorectal cancer cells.World J Gastroenterol. 2023 Jun 21;29(23):3606-3621. doi: 10.3748/wjg.v29.i23.3606. World J Gastroenterol. 2023. PMID: 37398890 Free PMC article.
-
ERK pathway activation contributes to the tumor-promoting effects of hepatic stellate cells in hepatocellular carcinoma.Immunol Lett. 2017 Aug;188:116-123. doi: 10.1016/j.imlet.2017.06.009. Epub 2017 Jun 28. Immunol Lett. 2017. PMID: 28668554
-
Ectodysplasin-A mRNA in exosomes released from activated hepatic stellate cells stimulates macrophage response.Exp Cell Res. 2022 Oct 15;419(2):113297. doi: 10.1016/j.yexcr.2022.113297. Epub 2022 Aug 11. Exp Cell Res. 2022. PMID: 35964664
-
Fluvastatin attenuates hepatic steatosis-induced fibrogenesis in rats through inhibiting paracrine effect of hepatocyte on hepatic stellate cells.BMC Gastroenterol. 2015 Feb 15;15:22. doi: 10.1186/s12876-015-0248-8. BMC Gastroenterol. 2015. PMID: 25886887 Free PMC article.
-
Cooperation of liver cells in health and disease.Adv Anat Embryol Cell Biol. 2001;161:III-XIII, 1-151. doi: 10.1007/978-3-642-56553-3. Adv Anat Embryol Cell Biol. 2001. PMID: 11729749 Review.
Cited by
-
The relationship between the tumor microenvironment of hepatocellular carcinoma - including cancer-associated fibroblasts and tumor-associated macrophages - and apparent diffusion coefficient.Am J Cancer Res. 2025 Apr 15;15(4):1747-1758. doi: 10.62347/ZGGJ9531. eCollection 2025. Am J Cancer Res. 2025. PMID: 40371143 Free PMC article.
References
-
- van de Velde CJ, Boelens PG, Borras JM, Coebergh JW, Cervantes A, Blomqvist L, Beets-Tan RG, van den Broek CB, Brown G, Van Cutsem E, Espin E, Haustermans K, Glimelius B, et al.. EURECCA colorectal: multidisciplinary management: European consensus conference colon & rectum. Eur J Cancer. 2014; 50:1.e–.e34. 10.1016/j.ejca.2013.06.048. - DOI - PubMed
LinkOut - more resources
Full Text Sources

