Colchicine Inhibits Neutrophil Extracellular Trap Formation in Patients With Acute Coronary Syndrome After Percutaneous Coronary Intervention
- PMID: 33346683
- PMCID: PMC7955504
- DOI: 10.1161/JAHA.120.018993
Colchicine Inhibits Neutrophil Extracellular Trap Formation in Patients With Acute Coronary Syndrome After Percutaneous Coronary Intervention
Abstract
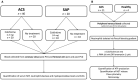
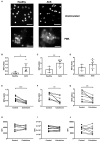
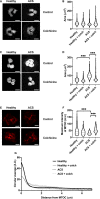
Background Release of neutrophil extracellular traps (NETs) after percutaneous coronary intervention (PCI) in acute coronary syndrome (ACS) is associated with periprocedural myocardial infarction, as a result of microvascular obstruction via pro-inflammatory and prothrombotic pathways. Colchicine is a well-established anti-inflammatory agent with growing evidence to support use in patients with coronary disease. However, its effects on post-PCI NET formation in ACS have not been explored. Methods and Results Sixty patients (40 ACS; 20 stable angina pectoris) were prospectively recruited and allocated to colchicine or no treatment. Within 24 hours of treatment, serial coronary sinus blood samples were collected during PCI. Isolated neutrophils from 10 patients with ACS post-PCI and 4 healthy controls were treated in vitro with colchicine (25 nmol/L) and stimulated with either ionomycin (5 μmol/L) or phorbol 12-myristate 13-acetate (50 nmol/L). Extracellular DNA was quantified using Sytox Green and fixed cells were stained with Hoechst 3342 and anti-alpha tubulin. Baseline characteristics were similar across both treatment and control arms. Patients with ACS had higher NET release versus patients with stable angina pectoris (P<0.001), which was reduced with colchicine treatment (area under the curve: 0.58 versus 4.29; P<0.001). In vitro, colchicine suppressed unstimulated (P<0.001), phorbol 12-myristate 13-acetate-induced (P=0.009) and ionomycin-induced (P=0.002) NET formation in neutrophils isolated from patients with ACS post-PCI, but not healthy controls. Tubulin organization was impaired in neutrophils from patients with ACS but was restored by colchicine treatment. Conclusions Colchicine suppresses NET formation in patients with ACS post-PCI by restoring cytoskeletal dynamics. These findings warrant further investigation in randomized trials powered for clinical end points. Registration URL: https://anzctr.org.au; Unique identifier: ACTRN12619001231134.
Keywords: acute coronary syndrome; inflammation; percutaneous coronary intervention; pharmacology.
Conflict of interest statement
None.
Figures

References
-
- Bekkers SCAM, Yazdani SK, Virmani R, Waltenberger J. Microvascular obstruction: underlying pathophysiology and clinical diagnosis. J Am Coll Cardiol. 2010;55:1649–1660. - PubMed
-
- Mangold A, Alias S, Scherz T, Hofbauer T, Jakowitsch J, Panzenbock A, Simon D, Laimer D, Bangert C, Kammerlander A, et al. Coronary neutrophil extracellular trap burden and deoxyribonuclease activity in ST‐elevation acute coronary syndrome are predictors of ST‐segment resolution and infarct size. Circ Res. 2015;116:1182–1192. - PubMed
-
- Carbone F, Mach F, Montecucco F. Update on the role of neutrophils in atherosclerotic plaque vulnerability. Curr Drug Targets. 2015;16:321–333. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

