Digital Monitoring and Management of Patients With Advanced or Metastatic Non-Small Cell Lung Cancer Treated With Cancer Immunotherapy and Its Impact on Quality of Clinical Care: Interview and Survey Study Among Health Care Professionals and Patients
- PMID: 33346738
- PMCID: PMC7781800
- DOI: 10.2196/18655
Digital Monitoring and Management of Patients With Advanced or Metastatic Non-Small Cell Lung Cancer Treated With Cancer Immunotherapy and Its Impact on Quality of Clinical Care: Interview and Survey Study Among Health Care Professionals and Patients
Abstract
Background: Cancer immunotherapy (CIT), as a monotherapy or in combination with chemotherapy, has been shown to extend overall survival in patients with locally advanced or metastatic non-small cell lung cancer (NSCLC). However, patients experience treatment-related symptoms that they are required to recall between hospital visits. Digital patient monitoring and management (DPMM) tools may improve clinical practice by allowing real-time symptom reporting.
Objective: This proof-of-concept pilot study assessed patient and health care professional (HCP) adoption of our DPMM tool, which was designed specifically for patients with advanced or metastatic NSCLC treated with CIT, and the tool's impact on clinical care.
Methods: Four advisory boards were assembled in order to co-develop a drug- and indication-specific CIT (CIT+) module, based on a generic CIT DPMM tool from Kaiku Health, Helsinki, Finland. A total of 45 patients treated with second-line single-agent CIT (ie, atezolizumab or otherwise) for advanced or metastatic NSCLC, as well as HCPs, whose exact number was decided by the clinics, were recruited from 10 clinics in Germany, Finland, and Switzerland between February and May 2019. All clinics were provided with the Kaiku Health generic CIT DPMM tool, including our CIT+ module. Data on user experience, overall satisfaction, and impact of the tool on clinical practice were collected using anonymized surveys-answers ranged from 1 (low agreement) to 5 (high agreement)-and HCP interviews; surveys and interviews consisted of closed-ended Likert scales and open-ended questions, respectively. The first survey was conducted after 2 months of DPMM use, and a second survey and HCP interviews were conducted at study end (ie, after ≥3 months of DPMM use); only a subgroup of HCPs from each clinic responded to the surveys and interviews. Survey data were analyzed quantitatively; interviews were recorded, transcribed verbatim, and translated into English, where applicable, for coding and qualitative thematic analysis.
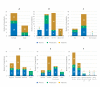
Results: Among interim survey respondents (N=51: 13 [25%] nurses, 11 [22%] physicians, and 27 [53%] patients), mean rankings of the tool's seven usability attributes ranged from 3.2 to 4.4 (nurses), 3.7 to 4.5 (physicians), and 3.7 to 4.2 (patients). At the end-of-study survey (N=48: 19 [40%] nurses, 8 [17%] physicians, and 21 [44%] patients), most respondents agreed that the tool facilitated more efficient and focused discussions between patients and HCPs (nurses and patients: mean rating 4.2, SD 0.8; physicians: mean rating 4.4, SD 0.8) and allowed HCPs to tailor discussions with patients (mean rating 4.35, SD 0.65). The standalone tool was well integrated into HCP daily clinical workflow (mean rating 3.80, SD 0.75), enabled workflow optimization between physicians and nurses (mean rating 3.75, SD 0.80), and saved time by decreasing phone consultations (mean rating 3.75, SD 1.00) and patient visits (mean rating 3.45, SD 1.20). Workload was the most common challenge of tool use among respondents (12/19, 63%).
Conclusions: Our results demonstrate high user satisfaction and acceptance of DPMM tools by HCPs and patients, and highlight the improvements to clinical care in patients with advanced or metastatic NSCLC treated with CIT monotherapy. However, further integration of the tool into the clinical information technology data flow is required. Future studies or registries using our DPMM tool may provide insights into significant effects on patient quality of life or health-economic benefits.
Keywords: advanced or metastatic non-small cell lung cancer; cancer immunotherapy; digital patient monitoring; drug- and indication-specific cancer immunotherapy module; eHealth; mHealth; patient-reported outcomes; quality of patient care; real-time symptom reporting; user experience.
©Oliver Schmalz, Christine Jacob, Johannes Ammann, Blasius Liss, Sanna Iivanainen, Manuel Kammermann, Jussi Koivunen, Alexander Klein, Razvan Andrei Popescu. Originally published in the Journal of Medical Internet Research (http://www.jmir.org), 21.12.2020.
Conflict of interest statement
Conflicts of Interest: All authors received support for third-party writing assistance for this manuscript, provided by F Hoffmann-La Roche Ltd, Basel, Switzerland. OS and BL have acted in an advisory and consultancy role for F Hoffmann-La Roche Ltd (payment planned). CJ and MK have received honoraria from, and have acted as external consultants for, F Hoffmann-La Roche Ltd. JA and AK are employees of, and hold shares and stocks in, F Hoffmann-La Roche Ltd. SI has acted in a consultancy and advisory role for Bristol-Myers Squibb, Roche, and Merck Sharp & Dohme; has participated in a speaker bureau or provided expert testimony for Boehringer Ingelheim; is an employee at an institution that has received a research grant or funding from Roche; and has received travel and accommodation expenses from Boehringer Ingelheim, Merck Sharp & Dohme, Roche, Novartis, and Kaiku Health. MK is a part-time contractor for F Hoffmann-La Roche Ltd. JK has received honoraria from AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Merck Sharp & Dohme, Novartis, Pfizer, Pierre Fabre, Roche, and Takeda; has acted in a consultancy and advisory role for AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Faron, Kaiku Health, Merck Sharp & Dohme, Novartis, Pfizer, Pierre Fabre, Roche, and Takeda; has received a research grant or funding from Roche; and has received travel and accommodation expenses from AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Faron, Kaiku Health, Merck Sharp & Dohme, Novartis, Pfizer, Pierre Fabre, Roche, and Takeda. MG is an employee at an institution that has received honoraria from F Hoffmann-La Roche Ltd. RP has acted in a consultancy and advisory role for Roche, Novartis, Merck Sharp & Dohme, Merck, Lilly, Bristol-Myers Squibb, AstraZeneca, Vifor Pharma, and Nutricia (all payments received by the institution) and is an employee at an institution that has received a research grant or funding from Roche, Novartis, Sanofi, and AbbVie.
Figures
References
-
- Perez-Moreno P, Brambilla E, Thomas R, Soria J. Squamous cell carcinoma of the lung: Molecular subtypes and therapeutic opportunities. Clin Cancer Res. 2012 May 01;18(9):2443–2451. doi: 10.1158/1078-0432.CCR-11-2370. http://clincancerres.aacrjournals.org/cgi/pmidlookup?view=long&pmid=2240... - DOI - PubMed
-
- National Comprehensive Cancer Network (NCCN) NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Non-Small Cell Lung Cancer. Version 7.2019. 2019. Aug 30, [2020-12-06]. https://tinyurl.com/ydgkgjv5.
-
- Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C, Mok TS, Reck M, Van Schil PE, Hellmann MD, Peters S, ESMO Guidelines Committee Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018 Oct 01;29(Suppl 4):iv192–iv237. doi: 10.1093/annonc/mdy275. https://linkinghub.elsevier.com/retrieve/pii/5115264 - DOI - PubMed
-
- Roche Registration GmbH Annex 1: Summary of product characteristics. European Medicines Agency. 2020. [2020-02-01]. https://www.ema.europa.eu/en/documents/product-information/tecentriq-epa....
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

